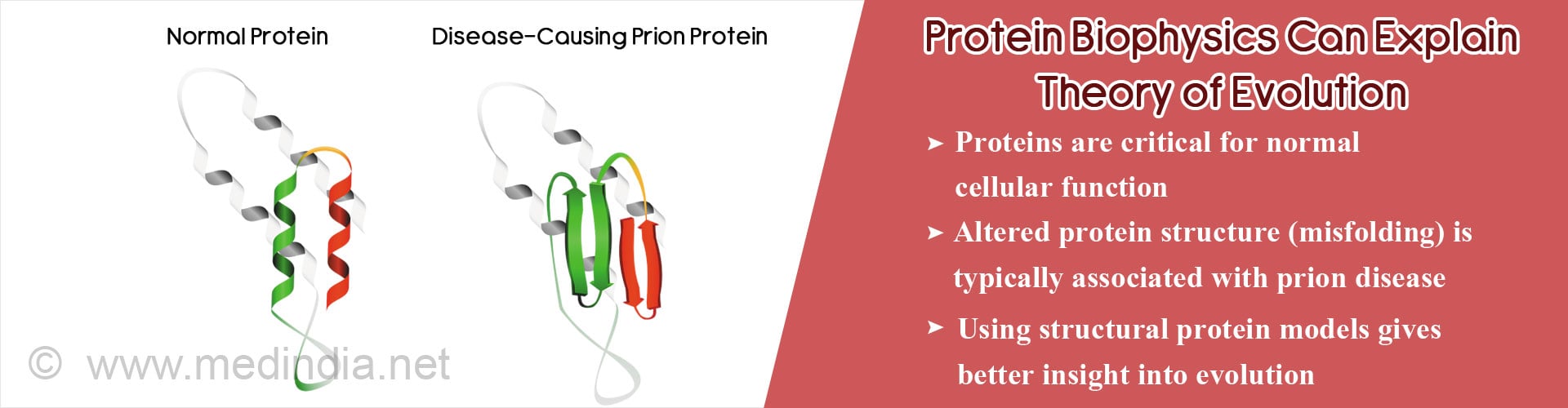
Biophysical protein models offer insight into role of protein structure, stability and binding affinities in phylogenetic evolution and causation of human disease.
- Misfolding (altered structure) of proteins and protein aggregation responsible for many diseases.
- In prion diseases, these misfolded protein aggregates are functional i.e. self-replicate, and move from cell to cell causing neurodegeneration.
- Misfolded protein models explain how altered protein structure might also have important functional implications, critical for phylogenetic evolution.
Reason for Study
Taking a cue from diseases caused by misfolding of proteins such as prion diseases ( rare neurodegenerative diseases caused by a type of protein which triggers the normal proteins in the brain), Biophysics scientists have used protein models to explain how alterations in the structure of proteins might- Explain creation and sustenance of life to keep in tune with the changing environment, a factor critical in the evolution of species over time.
- Help to determine if and whether controlling the process of protein folding and aggregation could one day prevent diseases caused by misfolding.
Methods of the Study
To test their hypothesis, the scientists generated a chemical system of peptides and coupled it to a physical system involving an alteration in the structure or other words misfolding of proteins.This combination ultimately led to gradual self-driven progressive changes in structure induced by the changing environment.
These protein polymers can fold into a seemingly endless array of forms, and sometimes behave like origami, Lynn explains. "They can stack into assemblies that carry new functions, like prions that move from cell-to-cell, causing disease."
Thus the protein models in the study inspired by the protein misfolding disease show how altered protein structure can bring about newer and useful functions as well which is critical for the phylogenetic evolution of species.
Although protein misfolding and aggregation is typically associated with disease, there are many instances in nature where these protein aggregates have beneficial purposes from a functional point of view. Some of these include
- Escherichia coli and other Enterobacteriaceae employ the rigid structure of amyloid aggregates to form fibrillar extracellular aggregates that promote bacterial adhesion, biofilm development and host invasion.
- In higher organisms such as the spidroin, amyloid aggregates, provide give spider silk a steel-like strength.
- Instances of functional amyloid aggregates have also been described in humans such as the amyloids of Pmel17 serving as a template for formation of melanin in mammalian melanocytes.
- An interesting example is the storage of peptide and protein hormones in the form of amyloid fibrils by the secretory granules in the pituitary gland.
- It has been postulated that almost all organisms may have used the toxic properties associated with protein aggregates to their advantage and developed defensive tools against pathogenic micro-organisms.
Protein Misfolding and Aggregates in Human Disease
Many neurodegenerative diseases such as Alzheimer’s and various prion and amyloid diseases are now known to be caused by protein misfolding or by aggregation of intermediate conformational states, with a tendency for misfolding enhanced by certain mutations. Cataracts in the human eye have also been shown to be caused by an accumulation of misfolded proteins and associated with mutations that increase the risk of misfolding.Cellular Mechanisms to Prevent Misfolding and Disease Causation
Cells have evolved mechanisms to prevent misfolding and aggregation of proteins, thereby preventing or reducing the occurrence of disease. Some of these include- Strict maintenance of protein concentration within the cell as this is shown to influence the formation of protein aggregates.
- Mechanisms such as chaperones that aid the protein folding process and reduce the chances of misfolding. They also reduce interactions of peptides with other molecules that may lead to aggregation and increase protein solubility within the cell.
- Creation and maintenance of an optimal environment to ensure correct folding of the proteins.
Role of Biophysics in the Health Sciences
Biological evolution employs genetic alterations or mutations as its basic working material. The current study of molecular evolution employs large amounts of protein sequence data, but only a relatively small body of protein structural data.- Research often fails to consider the impact of the structure and binding properties of peptides and amino acids in limiting the scope for evolution. Towards this end, a number of scientists from the biophysics community have recently called for a stronger interaction between the fields of Biophysics and Evolutionary Biology to understand the process of evolution better.
- Gaining better insight into protein structure and its impact on protein function can help in developing newer treatments or even prevention of protein misfolding diseases.
References:
- Neutral theory of molecular evolution - (https://en.wikipedia.org/wiki/Neutral_theory_of_molecular_evolution)
- Biophysics of protein evolution and evolutionary protein biophysics - (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4191086/)
- Biophysical models of protein evolution: Understanding the patterns of evolutionary sequence divergence - (http://biorxiv.org/content/biorxiv/early/2016/08/30/072223.full.pdf)
Source-Medindia