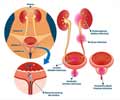
Treating urinary tract infections (UTIs) without the use of antibiotics may be in the horizon with the discovery of a novel chemical form of cellulose found in E.coli bacteria that sticks to the bladder cells.
- Escherichia coli (E. Coli) bacteria involved in urinary tract infections (UTIs) rely on a novel chemical form of the molecule cellulose called phosphoethanolamine (pEtN) cellulose to stick to bladder cells.
- pEtN cellulose is required to enhance the adhesion strength of bacteria with bladder epithelial cells
- pEtN cellulose can be targeted to treat UTIs and avoid the use of antibiotics that are increasingly becoming resistant to bacteria.
Phosphoethanolamine (pEtN) - Novel chemical form of cellulose
Cellulose is produced by plants, algae and some bacteria including E. coli. Being the most abundant organic polymer on Earth, it happens to be one of the best studied.Earlier, study co-leader Lynette Cegelski, an associate professor of chemistry at Stanford's School of Humanities and Sciences had surprisingly discovered a chemically unique form of cellulose, called pEtN (for the chemical group phosphoethanolamine) in the bacteria E. Coli.
Bacteria like E. coli, possess a biofilm, which is a slimy secretion, to share nutrients and to protect themselves against antibiotics and the immune system attacks of their host. The study showed that a crucial component of the biofilm was pEtN cellulose.
The research group in its next quest of measuring how strongly a layer of E. Coli bacteria sticks to a layer of isolated bladder cells sought the help of study co-leader Gerald Fuller, of the Fletcher Jones II Professor in the School of Engineering. Fuller had used an instrument called Live-cell monolayer rheometer (LCMR) to investigate the adhesion of the underbelly of the contact lenses to cells from the eye or the corneal cells.
Live-Cell Monolayer Rheometer (LCMR)
The LCMR works by maintaining a constant pressure between a glass plate and a single layer of live cells and then monitoring the cells with an attached microscope while keeping the cells alive with a heated bottom plate.Just by improving the microscopy, Fuller’s instrument could be used to better visualize the contact between the bacteria and bladder cell layer during measurements. This helped ensure that they always measured adhesion forces between these very thin layers.
They also required uniform layers of bacteria and bladder cells for obtaining reproducible LCMR measurements.
pEtN Cellulose – The Key Component
E. coli biofilms are created by braiding together pEtN cellulose and cell-surface fibers called curli that have been implicated in kidney infections and sepsis. In fact, the bacteria are more likely to make curli when the severity of the infections increases. UTIs are very common kidney infections and are mainly caused by E. coli.In trying to understand, the relative contributions of pEtN and curli to the adhesion of bacteria to host cells and how they worked together during infection, they designed a series of experiments that tested the adhesion strength of the curli and the cellulose separately as well as together.
First, they chose E. coli whose biofilms contained both cellulose and curli and attached these to the top plate of the LCMR and then brought it into contact with a bottom plate containing bladder cells. The scientists then quickly sliced a tiny amount of the top plate horizontally, to obtain a quantitative measure of the bacteria's "stickiness" using the resulting level of adhesive stress.
The procedure was repeated with genetically engineered E. coli whose biofilms contained only cellulose or only curli.
The results showed that cellulose acted like glue and helped the bacteria to adhere strongly to the cells. The highest adhesion strength was exhibited by the bacteria that produced both curli and cellulose, followed by the curli alone, and lastly the cellulose alone.
The results suggest that the cellulose can be attacked to prevent bacterial adhesion and in turn break the cycle of infection. This will be an excellent alternative to traditional antibiotics and a way to avoid drug resistance.
Source-Medindia