Experimental anti-tuberculosis drug named AN12855 found to be more effective and significantly shortens the duration of treatment compared to standard isoniazid treatment currently used.
- Experimental anti-tuberculosis drug AN12855 shown to be more effective than standard drug isoniazid in treating tuberculosis and also significantly shortens treatment duration
- According to WHO estimates, tuberculosis remains the main infectious cause of death globally; more than 10 million people were diagnosed with tuberculosis resulting in 1.6 million deaths
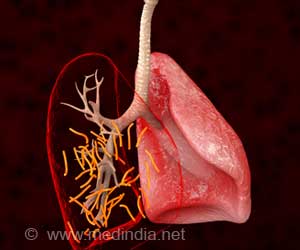
- Mycobacterium tuberculosis (M. tuberculosis) is the organism that causes tuberculosis (TB) in humans mainly involving the lungs, although it can attack other parts of the body as well
Read More..
Developing Newer & Effective Anti-Tuberculosis Drug - AN12855
The goal of the study team was to develop newer and more effective universal treatment regimens for tuberculosis to combat emerging multidrug resistance and decrease treatment duration which is currently for at least a minimum period of six months and can go up to a year or more.The experimental drug AN12855 was developed keeping these goals in mind.
AN12855 Superior to Isoniazid in Efficacy on Drug Testing
The study team tested the efficacy of the drug AN12855 against the standard drug, isoniazid. The major advantages of AN12855 included the following -1. Lesser Risk of Drug Resistance with AN12855
Isoniazid (INH) drug requires an enzyme produced by the mycobacterium called KatG to be converted to its active form and enable the killing of the mycobacterium. Some mycobacteria do not produce this enzyme and thus isoniazid becomes ineffective. The absence of the enzyme, however, does not affect the Pathogenicity of the mycobacterium or its capacity to multiply and damage the host tissue. The absence of the KatG enzyme increases the chances of development of drug resistance. AN12855 is effective even in the absence of this enzyme
2. AN12855 Drug More Effective in Sites Where Mycobacteria Are Highest in Number
One of the hallmarks of tuberculosis infection is a host tissue reaction leading to the formation of well circumscribed spherical bodies called granulomas within which the mycobacteria are confined. Often it is difficult for drugs to penetrate the granuloma to reach the organism
Not many studies until now have replicated this advanced stage of disease in animal models while testing for drug efficacy. In the current study, the team used a specialized mouse model in which these granulomas were produced and then both the drugs were tested to replicate the tissue reaction in patients with clinically active TB.
The findings proved that AN12855 was superior to isoniazid in entering the granulomas and killing the mycobacteria. The additional advantage was the decreased development of drug resistance.
"We discovered that the drugs differed dramatically with respect to their abilities to kill the pathogen in highly diseased tissues," said Dr. Robertson. AN12855 proved more effective, "without selecting for appreciable drug resistance," said Dr. Robertson
The findings of the study confirm that the experimental drug AN12855 is superior to standard isoniazid in penetrating sites where bacteria are in high concentration, killing of bacteria as well as decreased development of drug resistance.
Scope of the Study
- Paves the way for the development of antituberculosis drugs that shorten treatment duration
- Using a specialized TB mouse efficacy model (dubbed C3HeB/FeJ) as a research tool to simulate advanced disease stages in humans and testing the efficacy of drugs in advanced disease states
Summary
Experimental drug AN12855 superior to standard drug isoniazid in killing bacteria along with lesser chances of development of drug resistance by the mycobacteria.Reference:
- Efficacy and improved resistance potential of a cofactor-independent InhA inhibitor of Mycobacterium tuberculosis in a C3HeB/FeJ mouse model - (https://aac.asm.org/content/early/2019/01/24/AAC.02071-18)
Source-Medindia