Chimeric Antigen Receptor (CAR) T cell therapy has shown some promising results in multiple myeloma treatment option in patients who have had a relapse or were resistant
Highlights
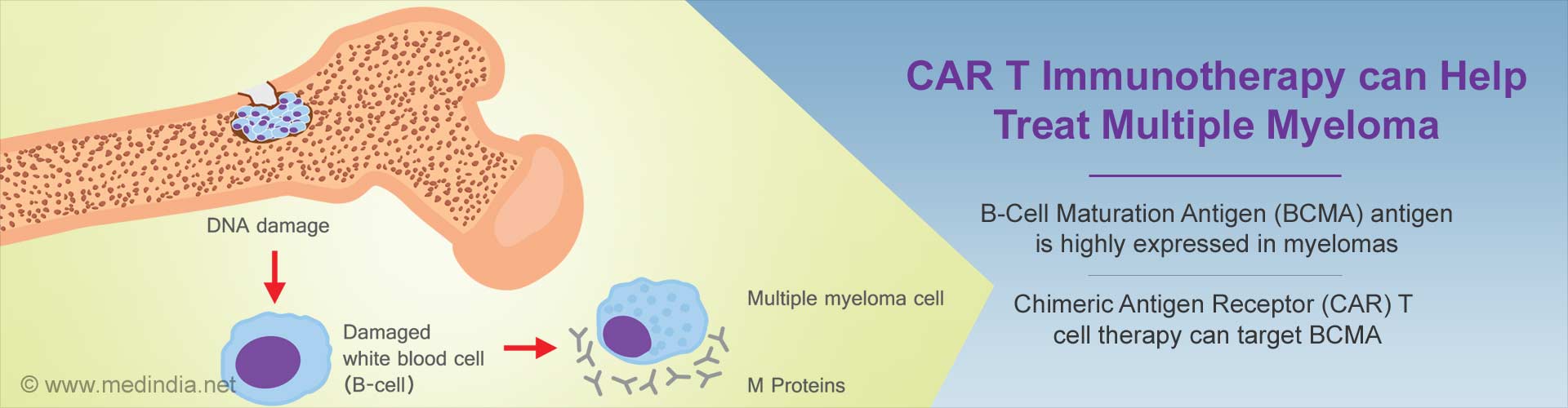
- Chimeric Antigen Receptor (CAR) T cell therapy can now target a receptor called B-Cell Maturation Antigen (BCMA), which is highly expressed in myeloma cancer patients
- Multiple Myeloma (MM) is a bone marrow cancer that affects plasma cells making them grow out of control and become cancerous
- CAR T cell therapy can reprogramme patients’ own immune T cells to destroy cancer cells. Once infused into the patients, they gain the ability to multiply and target cells that express BCMA
Both of these investigational approaches targeted a receptor called B-Cell Maturation Antigen (BCMA), which is highly expressed in myeloma and thus a promising target for treatment. These studies will be presented as oral abstracts at the 59th Annual American Society of Hematology Meeting and Exposition in Atlanta.
Multiple Myeloma (MM) is a bone marrow cancer that affects plasma cells. Normal plasma cells work as part of the immune system, but in MM these cells become cancerous and grow out of control, leading to multiple painful bone tumors, as well as anemia, kidney failure and recurrent infections.
The American Cancer Society estimated there will be more than 30,200 new cases of MM in 2017. The Standard treatments include chemotherapy, radiation and sometimes a stem cell transplant.
The first study (Abstract #505) used CART-BCMA, a specifically engineered type of CAR T cell developed by Penn researchers in collaboration with Novartis as part of a global research and development alliance that began in 2012.
Patients were given a single dose of chemotherapy before the CART-BCMA infusion to temporarily clear out normal white blood cells and help the hunter cells to expand. Two dose levels of CART-BCMA cells were explored in a population of heavily-pretreated patients. These patients had a median of seven prior lines of therapy.
Twelve of the 15 patients experienced cytokine-release syndrome, a toxicity that involves varying degrees of flu-like symptoms, with high fevers, nausea, and muscle pain, and can require ICU-level care. All patients recovered, with one requiring tocilizumab, a standard therapy for this side effect, and one receiving siltuximab, a similar cytokine-blocking drug.
Three of the patients in the study experienced neuro-toxicity, which resolved on its own. There were no treatment-related deaths.
"This follows up on our previous report that showed that six out of nine patients had clinical benefit to CART-BCMA given alone, without chemotherapy," said the study’s lead author Adam D. Cohen, MD, an assistant professor of Hematology and Oncology at Penn and the director of Myeloma Immunotherapy in Penn’s Abramson Cancer Center.
"All of these data indicate BCMA is an attractive target in refractory myeloma, and our hope is that combining the CAR T cells with chemotherapy will increase the expansion and persistence of the CAR T cells, though longer follow-up is needed to assess this."
Michael C. Milone, MD, PhD, an associate professor of Pathology and Laboratory Medicine at Penn, was the study’s senior author. The study was supported by the Penn-Novartis alliance.
Cohen is the senior author on a second study also targeting BCMA in MM (Abstract #741), in this case with an experimental drug called GSK2857916, for which the FDA granted a breakthrough therapy designation in early November to allow for expedited development.
It links a chemotherapy drug called MMAF to a monoclonal antibody against BCMA, allowing for targeted delivery of the chemotherapy directly to the myeloma cancer cells in the bone marrow.
In this first-in-human, multi-center trial, patients received an infusion every three weeks for up to 16 treatment cycles. The overall response rate was 60 percent (21 out of 35 patients), including 51 percent with very good partial responses meaning more than a 90 percent reduction in myeloma protein levels. The median progression-free survival was about eight months.
"Response rates that high are really unprecedented for a single agent in relapsed, refractory disease," Cohen said. "It’s especially important to this patient population, since they are heavily pre-treated and have limited options for treatment."
Cohen noted 57 percent of patients in this trial had undergone five prior lines of therapy or more. There was significant eye toxicity associated with this treatment, including dry eye, blurry vision, and light sensitivity in 63 percent of patients. Cohen said these effects were caused by the chemotherapy portion of the drug. They were mild in most patients and improved with eye drops.
These patients held treatment and restarted at a lower dose once the toxicity resolved. Cohen says Penn will continue research related to this compound. There is a larger study planned around the treatment, as well as a study evaluating this drug in combination with other therapies.
Reference
-
Adam D. Cohen, Alfred L. Garfall, Edward A Stadtmauer, Simon Francis Lacey, Eric Lancaster, Dan T. Vogl, Karen Dengel, David E Ambrose, Fang Chen, Gabriela Plesa, Irina Kulikovskaya, Vanessa E Gonzalez, Minnal Gupta, Regina M Young, Tenesia Carey, Regina Ferthio, Brendan M. Weiss, Celeste Richardson, Randi E. Isaacs, J. Joseph Melenhorst, Bruce L. Levine, Carl H June, Michael C. Milone: B-Cell Maturation Antigen (BCMA)-Specific Chimeric Antigen Receptor T Cells (CART-BCMA) for Multiple Myeloma (MM): Initial Safety and Efficacy from a Phase I Study Blood (ASH Annual Meeting) 128: #1147
Source-Eurekalert