Small compounds to correct mitochondrial dysfunction that leads to Charcot-Marie-Tooth disease and other conditions involving mitochondria.
- Charcot-Marie-Tooth Disease is the most common inherited neurologic disorder
- No drugs are available to slow or stop Charcot-Marie-Tooth Disease progression
- Small peptide inhibitors may help correct mitochondrial dysfunction
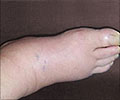
Charcot-Marie-Tooth (CMT) Disease
- It is an inherited disorder caused by the genetic mutations that disrupt mitochondria
- The disease leads to gradual loss of motor neurons and eventually result in paralysis
- CMT affects nearly 3 million people globally
- Symptoms are visible between the ages 10 and 20
- Onset of symptoms before 10 years of age is serious and patients may require crutches or a wheelchair The life span of patients with CMT is average but patients gradually lose motor control of the legs.
Mitofusin 2 protein has gained huge attention because scientists think the protein has a role in many diseases including diabetes and cardiovascular disease. Mitofusin 2 protein governs whether two mitochondria are able to tether to each other and then fuse, exchanging genetic information, which is thought to be important for maintaining healthy mitochondria and, by extension, healthy tissues.
"In the past, scientists assumed mitofusin 2 was always active, always ready to tether to another mitofusin molecule and promote mitochondrial fusion," Dorn said. "Our study now shows this is incorrect. Mitofusin 2 folds and unfolds, giving it active and inactive forms that either encourage or discourage tethering and the resulting fusion of mitochondria."
Once the scientists have understood how mitofusin 2 changes shape, they were able to design small peptides that interact with the protein and drive it toward either an active or inactive state.
Role of Peptide Inhibitors
TetherX another small molecule forces mitofusin 2 into its inactive state, which suppresses tethering and prevents fusion.
"The design of these peptide inhibitors was a challenge," Mochly-Rosen said. "But it is always exciting when a basic research discovery leads to the design of a new drug that may eventually help patients who currently have no treatment options."
Researchers hope that GoFuse, or a similar molecule, could encourage the mitochondrial tethering and fusion that is missing in Charcot-Marie-Tooth disease. If such tethering could be restored, it could prevent or delay the loss of motor neurons that gradually paralyzes many patients with this genetic disorder.
"Re-establishing oxygen flow is really important after a heart attack or stroke," Dorn said. "But you also get a huge wave of cell death when oxygen suddenly returns to tissues of the body, such as the heart or the brain."
"These peptides are two sides of the same coin," Dorn said. "Mutations that disrupt tethering cause a neurodegenerative disease. We would like to encourage tethering in that case. But there are other situations where tethering is destructive, and we would like the ability to interrupt it briefly and then go back to normal. We’ve shown these peptides can influence mitochondrial tethering in cells grown in the lab, and now we are working to test them in mouse models of disease."
Source-Medindia