The United States Food and Drug Administration granted orphan drug designation to Yeliva drug for Cholangiocarcinoma treatment.

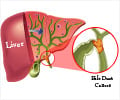
- Cholangiocarcinoma or the cancer of the bile ducts is the second most common liver cancer in the world.
- The Food and Drug Administration (FDA) grants Orphan drug designation for Yeliva drug to treat Cholangiocarcinoma.
Cholangiocarcinoma or bile duct cancer is a lethal malignancy condition with a very fewer treatment options. In the United States, around 8000 people are diagnosed with bile duct cancers. Treatments are not effective and cholangiocarcinoma has a poor prognosis.
The five-year survival rate of intrahepatic and extrahepatic cholangiocarcinoma patients may range between 2% to 30%
Clinical Trials
The research study was conducted on three patients with cholangiocarcinoma in Phase I study. These patients had prior therapy. Of these patients, one of them had sustained partial response and the other two patients had prolonged stable disease.
The study will evaluate Yeliva drug as a single agent in cholangiocarcinoma patients which will determine single response rate for treatment.
Several phase I and phase II studies with Yeliva drug are ongoing for the treatment of advanced hepatocellular carcinoma.
Yeliva drug is an orally administered sphingosine kinase-2 (SK2) inhibitor. The drug mainly acts by inhibiting the SK2 enzyme and blocks the synthesis of sphingosine 1 -phosphate (S1P). This lipid molecule may promote cancer growth and pathological inflammation.
The drug is well-tolerated and can be safely administered to patients at doses which provide circulating levels.
Orphan drug designation is assigned for drugs which are used for the treatment of rare disease or condition upon the request of the sponsor.
Benefits of Orphan Drug Designation
- Includes Tax Credit for Qualified Clinical Testing
- Waiver for Prescription drug user fee (PDUFA Fee)
- Seven-year marketing exclusivity period for Cholangiocarcinoma treatment if the drug is approved.
- It is a type of liver cancer which arises within the biliary system.
- Cholangiocarcinoma is often referred to as the cancer of the bile ducts.
- There is no early diagnosis for cholangiocarcinoma.
- Cholangiocarcinoma is more common in people above the age of 60 years.
- Symptoms of cholangiocarcinoma may include abdominal discomfort, loss of appetite, tiredness and weight loss.
- Cholangiocarcinoma: The facts - ( http://worldcholangiocarcinomaday.org/item-2/)
Source-Medindia