Fecal Immunochemical Testing (FIT) is usually preferred by people for screening of colorectal cancer. But a higher number of lesions can be detected more clearly using colonoscopy.
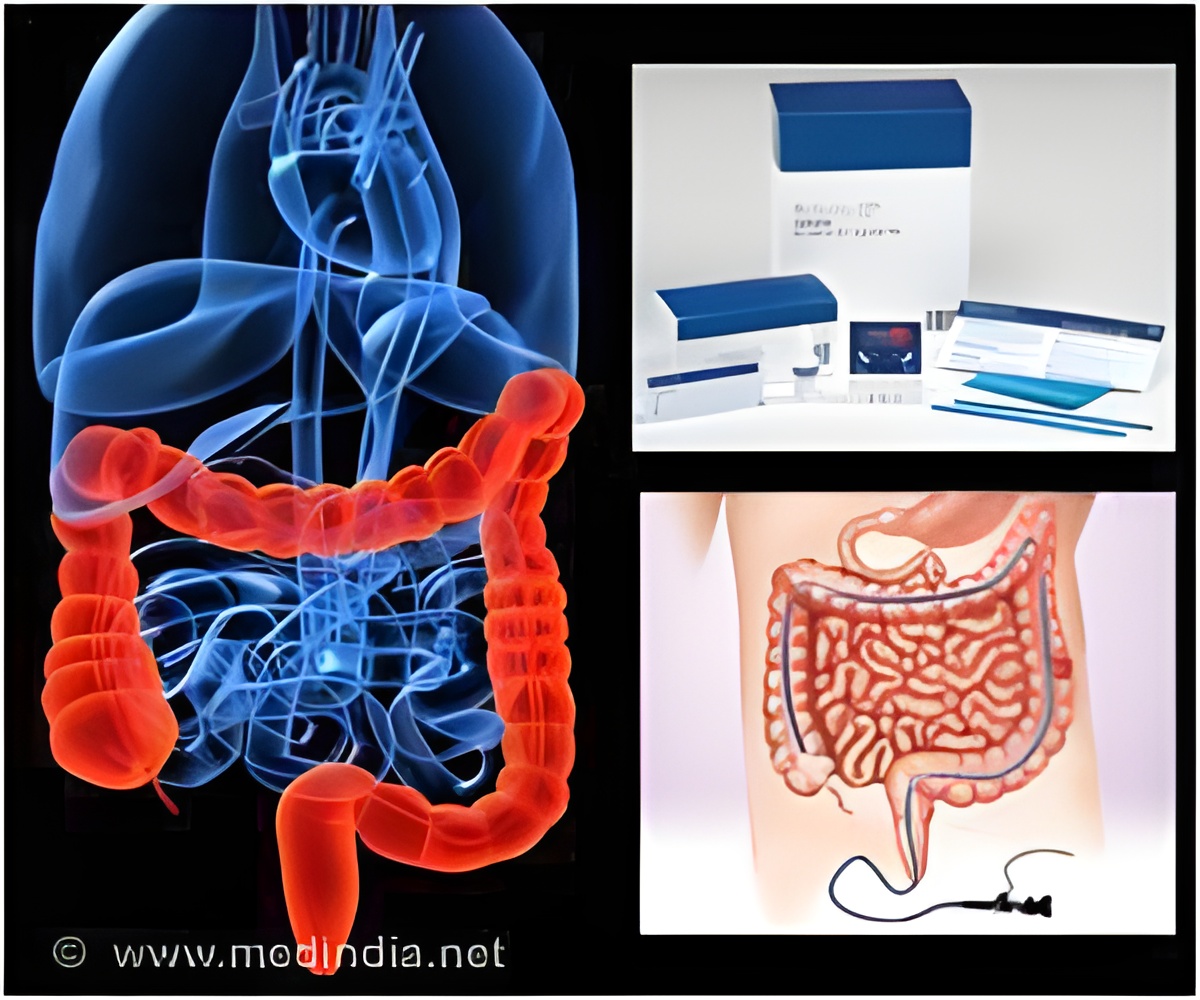
The non-invasive tests for the detection of colorectal cancer include the Fecal Immunochemical testing (FIT), fecal occult blood testing (FOBT) and stool DNA testing. The more invasive screening tests include colonoscopy, sigmoidoscopy and barium enema.
The fecal immune-chemical testing (FIT) is a non-invasive test which includes an at-home collection of stool samples, which are then lab tested mainly for detection of the presence of any blood in the feces. Blood in the feces is not always visible with naked eyes and is thus tested chemically. The presence of blood may be suggestive of a cancerous lesion in the colorectal area. However, feces can test positive for blood even due to other causes such as gastrointestinal bleeding, ulcers, adverse drug reaction, use of NSAIDS etc.
Colonoscopy is an invasive method of detection where a long tube with a small camera at the end is inserted through the anus into the colon and the areas of colon are viewed on the screen. The procedure usually is done with the help of sedatives in order to reduce the discomfort to the patient. Areas of abnormal growth in the colon can be removed for testing during this procedure. A more clear and elaborate view of small and big cancerous growths is obtained with this procedure.
A positive outcome of fecal-immunochemical testing (FIT) usually calls for a detailed testing with colonoscopy. This is done with the aim to precisely detect the presence of colorectal cancer (if any) and the extent of its spread. A recent study compared colonoscopy with FIT in the screening of colorectal cancer amongst the average-risk population.
Published in the New England Journal of Medicine, the study was conducted on adults between the age group of 50-69 years who were free of any symptoms of colorectal cancer. A comparison was made between 26,703 adults screened once for colorectal cancer using colonoscopy with 26,599 adults who were screened using FIT every two years.
The researchers of the study concluded that a higher participation rate was seen amongst the group undergoing FIT. Though the number of cases of colorectal cancer detected was similar in both the groups, a higher number of adenomas were found in the group undergoing colonoscopy.
Source-Medindia