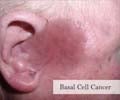
Vismodegib (GDC-0449) a hedgehog pathway inhibitor evoked a 30-43% response rate, over an average of 7.6 months, in basal-cell carcinoma patients - the most common type of non-melanoma cancer.
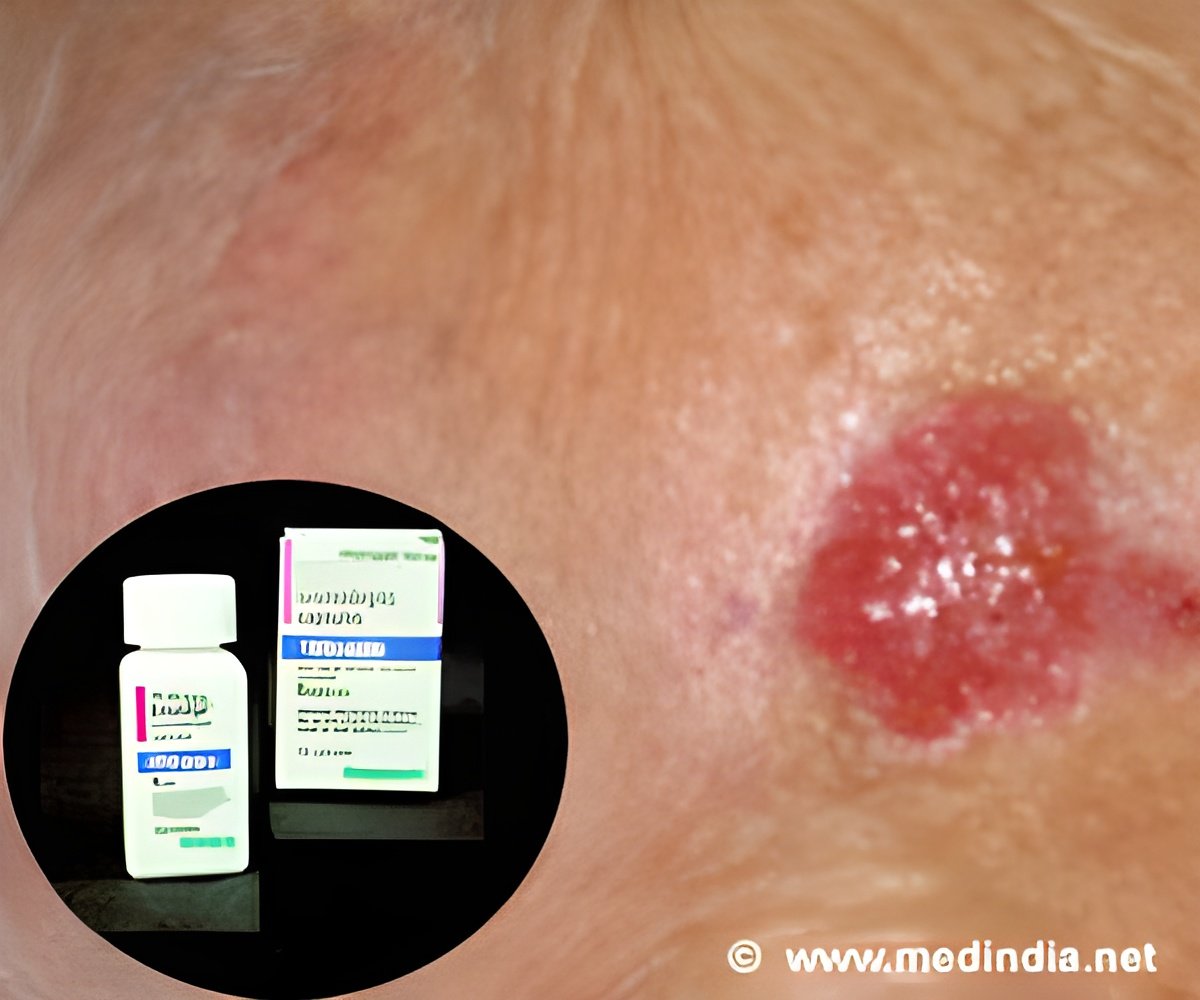
A phase 1 study with vismodegib, a small-molecule drug from Genentech, comprising of 33 advanced basal-cell carcinoma patients revealed a 58% response rate for an average of nearly 13 months.
The current phase 2 study, in basal-cell carcinoma patients, was designed to further assess vismodegib efficacy and safety. Metastatic basal-cell carcinoma (33) and locally advanced inoperable basal-cell carcinoma (71) patients were enrolled (total 104) across multiple centers (31) and countries (3). Eligible patients were ≥18 years with acceptable organ function and an ECOG performance status of ≤2. About 30%, 58% and 97% metastatic basal-cell carcinoma patients had previously received systemic, surgical and radiation treatment respectively. Nearly 11%, 27%, 21% and 89% locally advanced basal-cell carcinoma patients had previously received topical, systemic, surgical and radiation treatment respectively.
There was no control group in this study and all patients were administered daily dose of 150 mg oral vismodegib for 13 months or until disease progression (20% increment or new lesion) or toxicity was observed. Simultaneous antitumor therapy was disallowed.
The primary end point was the response rate, at least 30% decrease in outward or radiographic evidence of the carcinoma (tumor shrinkage), such that response rate was expected to be ≥20% for locally advanced basal-cell carcinoma patients and ≥10% for metastatic basal-cell carcinoma patients. Response time was a key secondary end point.
Tumors were assessed radiographically, at week 0 and every 8 weeks, or by physical inspection using the Response Evaluation Criteria in Solid Tumors (RECIST) guidelines. Adverse events were recorded for 45 days after the final drug dose or discontinuation from the trials. Expression of GLI family zinc finger 1 (GLI1) and patched homologue 2 (PTCH2), hedgehog target genes, was evaluated by PCR (polymerase-chain-reaction assay).
Almost 50% patients withdrew from the study such that average drug treatment was for 10 months. Adverse events appeared in all patients with grade 1-2 side effects occurring in 57% treated patients. Over 30% patients were recorded to have muscle spasms, alopecia (hair loss), dysgeusia (taste disorder), weight loss and fatigue. Nearly 25% patients suffered from serious adverse events including death in 7 patients.
Reference: Efficacy and Safety of Vismodegib in Advanced Basal-Cell Carcinoma; Aleksandar Sekulic et al; N Engl J Med 2012; 366:2171-2179.
http://www.nejm.org/doi/full/10.1056/NEJMoa1113713
Source-Medindia