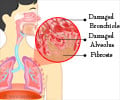
Cells activated by autocrine pathway produce excess collagen that leads to progressive scarring and rejection of transplanted lungs.
- Survival rate of lung transplantation is as low as 50% in five years.
- This is because of a process called bronchiolitis obliterans syndrome (BOS) in which cells produce excess collagen leading to complete scarring of lung tissue.
- The cells are kept activated by a chain of upstream signals starting with autotaxin, an enzyme generating lysophosphatidic acid, which signals more collagen production, hastening the scarring process.
"Survival of lung transplantation is worse than all other solid organ transplants," she says. "The five-year survival rate is only 50%, and the 10-year survival rate is as low as 20%. For me to tell my patient that this second chance at life comes with this critical limitation is incredibly hard."
"Small airways of the transplanted lung, or graft, begin scarring and slowly become completely scarred and close up. This process is called bronchiolitis obliterans syndrome (BOS)," she says.
"The patient will begin to have shortness of breath again, like they did before the transplant, and this scarring can lead to graft problems and ultimately death in some patients. Right now we have nothing to prevent or stop this scarring process once it begins." Lama added.
Studying the Scarring Process
"This study is unique because it actually started from our patients," Lama says. "Samples were collected from lung transplant patients by going into the lung with a small scope that helps us to insert a liquid into the lung and draw it back, allowing us to study the internal environment of the transplanted organ. This procedure, called bronchoalveolar lavage, is routinely done to rule out acute rejection or infection."
"We started investigating cells in patients who have BOS and found that even after being removed from the fibrotic graft, these cells stayed activated, making more collagen, which explained their ability to promote relentless scarring," Lama says.
On further analysis, it was revealed that a chain of upstream signals starting with autotaxin, an enzyme which acts on the cell membrane to generate lysophosphatidic acid, is what kept the cells activated.
This potent lipid mediator was signaling the cells to produce more collagen, as well as indirectly increased autotaxin levels.
"We found that these cells could regulate themselves by increased autotaxin production, which was being further enhanced by an autocrine loop," Lama says.
"What’s so fascinating about this is that it means the cell no longer needs an inflammatory environment, or stimulation in its environment, to produce the collagen. That’s extremely novel because we have never thought of these cells as essentially cancer cell-like in nature, but they are regulating their own behavior like a cancer cell does." Lama added.
She adds, "The dysregulated behavior of these cells essentially makes them become autonomous in behavior and helps us further understand why we can’t stop the scarring process just by changing the environment around the cell, which does not make a difference. They have already begun not listening to anything around them."
Finding New Targeted Therapies
This led the team to investigate new therapies.
"If we interrupt this pathway, we could potentially stop further development of lung scarring and save the graft," she says.
Researchers found two new therapies that could help interrupt this pathway:
- PF-8380 that targeted the enzyme
- AM095 that targeted the receptor for lysophosphatidic acid
Researchers found that when mouse models with chronic rejection, were orally treated with these drugs, they were protected from the scarring process of chronic rejection.
The team hopes that these findings will pave way for future clinical trials.
"Drugs targeting this pathway, such as autotaxin inhibitors and LPA1 receptor antagonists, have already been developed and are in clinical trials for other fibrotic conditions of the lung, such as idiopathic pulmonary fibrosis," Lama explains. "Now we hope that we can consider similar therapies in BOS, a disease where we have no therapeutic options."
"These findings suggest that understanding the pathways that activate a mesenchymal cell and targeting them is crucial if we want to contain the progression of BOS," Lama says. "We hope this work, which started from the bedside when we examined the lavage fluid from our lung transplant recipients, will be able to be translated back from the bench to the bedside to make an impact on the lives of our patients."
The paper is published in the Journal of Clinical Investigation.
Reference
- Vibha Lama et al. Autocrine lysophosphatidic acid signaling activates β-catenin and promotes lung allograft fibrosis. Journal of Clinical Investigation; (2017) doi.org/10.1172/JCI88896.
Source-Medindia