The US Food and Drug Administration warned clinicians that excessive doses of Ocaliva for patients with liver disease can result in serious injury or death.
Highlights
- The FDA releases black box warning for the drug Ocaliva after the excessive dosing.
- Incorrect dose of Ocaliva for a rare liver disease, increases the risk of liver injury and death. /
- The drug manufacturer, Intercept Pharmaceuticals Inc has released prescribing information for Ocaliva to health care providers.
Nineteen deaths and 11 cases of serious liver injury were associated with the use of Ocaliva, the FDA said. Some patients were receiving higher doses of the drug, particularly due to a higher frequency of dosing than recommended in the label, the agency added.
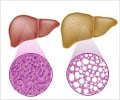
Ocaliva For Liver Disease
Ocaliva is used to treat a rare, chronic liver disease known as primary biliary cholangitis (PBC). PBC causes the bile ducts in the liver to become inflamed, damaged and destroyed. This causes bile, a fluid that helps in digestion, to build up in the liver. This build-up damages the liver over time, eventually causing it to lose its ability to function.
The FDA approved obeticholic acid in May 2016 to treat adults with PBC in combination with ursodeoxycholic acid (UDCA) when the latter drug falls short therapeutically, or by itself when adults can’t tolerate UDCA.
Adverse Symptoms of Ocaliva
- New or worsening fatigue
- Diarrhea
- Weight loss
- Abdominal pain
- Decreased appetite
- Nausea and vomiting
- Change in behavior or confusion
- Vague symptoms such as anxiety or unease
- Abdominal swelling
- Yellow eyes or skin
- Steatorrhea or Bloody stools
Reference
https://www.fda.gov/Drugs/DrugSafety/ucm576656.htm.