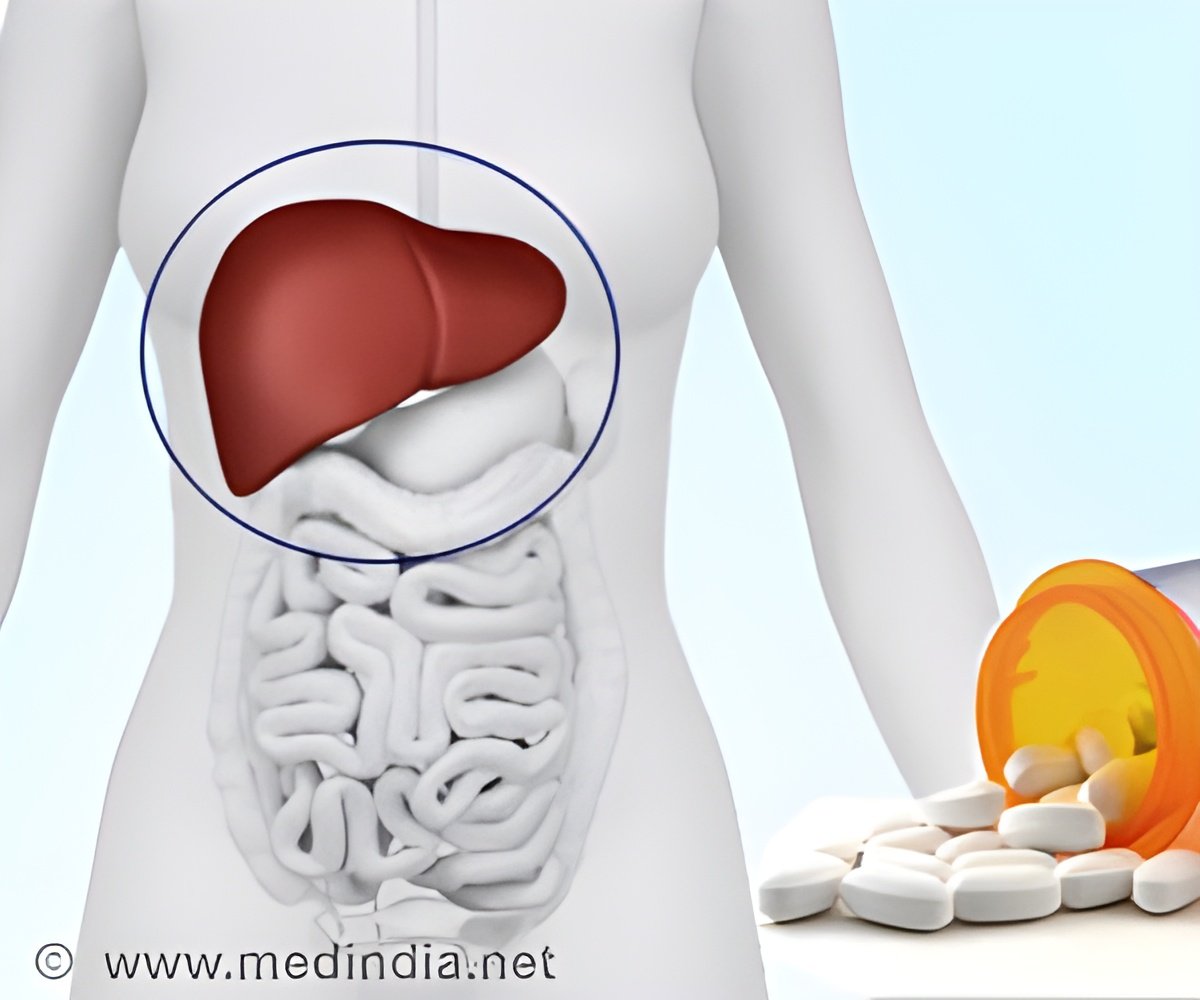
The US Food and Drug Administration (FDA) has recently granted approval for interferon-free treatment for those affected with hepatitis C genotype 1.
The Treatment:
The combination of 2-3 direct-acting antiviral agents (DAAs) will be able to result in 90 percent sustained virologic response (SVR) rates mostly in patient with genotype 1 infection. The list includes patients with subtype 1a, treatment-experienced patients and patients with advanced liver disease - cirrhosis. Some of these regimens may be ribavirin (RBV) free. It is incredible news to most patients as their wait is almost over, especially, for those with genotype 1 infection.
The Remedy:
The drug Viekira Pak treats patients with chronic hepatitis C virus (HCV) genotype 1 infection. This also includes those with cirrhosis, a type of advanced liver disease. Viekira Pak includes - ombitasvir, paritaprevir and dasabuvir – the three new drugs that inhibit growth of HCV. It includes a previously approved drug – ritonavir, which helps increase the blood levels of paritaprevir. For patients with unstable functioning of the liver, Viekira Pak intake is recommended along with ribavirin. For others, Viekira Pak can be consumed with or without ribavirin.
Researchers Say…
“The new generation of therapeutics for hepatitis C virus is changing the treatment paradigm for Americans living with the disease,” said Edward Cox, M.D., M.P.H., The Director, Office of Antimicrobial Products in FDA’s Center for Drug Evaluation and Research. “We continue to see the development of new all-oral treatments with very high virologic response rates and improved safety profiles compared to some of the older interferon-based drug regimens.”
Clinical Trials:
The effectiveness of Viekira Pak was estimated through six clinical trials with 2.308 participants. All participants were affected by chronic HCV infection, some with cirrhosis and some without cirrhosis. The participants were given Viekira Pak with or without ribavirin for a 12 to 24 weeks period. Trials measured if HCV was no longer found in blood at least 12 weeks after treatment. Multiple population results revealed that 91 to 100 percent participants who were given Viekira Pak in recommended dosages accomplished SVR.
Hepatitis C – The Viral Disease:
HCV causes inflammation of the liver and reduces liver function. It may also lead to liver failure or liver cancer. Many of them infected with Hepatitis C diagnose only at the condition of liver damage and this can take decades. Apparently there are no symptoms for the infection. The Centers for Disease Control and Prevention reveals that HCV affects about 3.2 million Americans. About 15 to 30 precent of them developed cirrhosis due to lack of treatments.
Source-Medindia