Centers for Disease Control and Prevention recommends two dose -HPV vaccine schedule for preventing cervical cancer and infections in preteens.
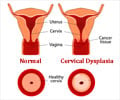
- Human Papilloma virus (HPV) infection leads to cervical cancer and genital warts.
- Centers for Disease Control and Prevention recommends two shots of HPV vaccine for young adolescents.
- The U.S. Food and Drug Administration also approved the two dose HPV vaccine schedule for preteens.
Tom Frieden, M.D., M.P.H., CDC Director said ,“Safe, effective, and long-lasting protection against HPV cancers with two visits instead of three means more Americans will be protected from cancer,” “This recommendation will make it simpler for parents to get their children protected in time.”
According to the Centers for Disease Control and Prevention, around 80 million Americans are affected by HPV infections.
Preteens usually receive HPV along with whooping cough and meningitis vaccine. Two doses of HPV vaccine at the age of 11 and 12 with six months gap may help in the prevention of infections.
The Advisory Committee on Immunization Practices (ACIP) is a panel of experts who advise CDC on vaccine recommendations in the United States. They have voted for a 2 dose schedule of vaccine for infections. CDC has approved the recommendations of ACIP for HPV vaccine schedule and the agency guidelines were published in the Morbidity and Mortality Weekly Report (MMWR).