From curiosity to groundbreaking cure: How a team in India developed a game-changing cancer therapy. You won't believe the results!
- A collaborative effort led by Alka Dwivedi in India resulted in the development of NexCAR19, India's first approved CAR T-cell therapy, addressing the accessibility and affordability concerns of cancer treatment
- The therapy showed promising results in clinical trials, with a significant decrease in cancer extent and minimal side effects, marking a new era in cancer treatment in India
- Through innovation, collaboration, and perseverance, the team overcame challenges to create a homegrown solution tailored to the needs of Indian patients, setting a precedent for global cancer research and treatment
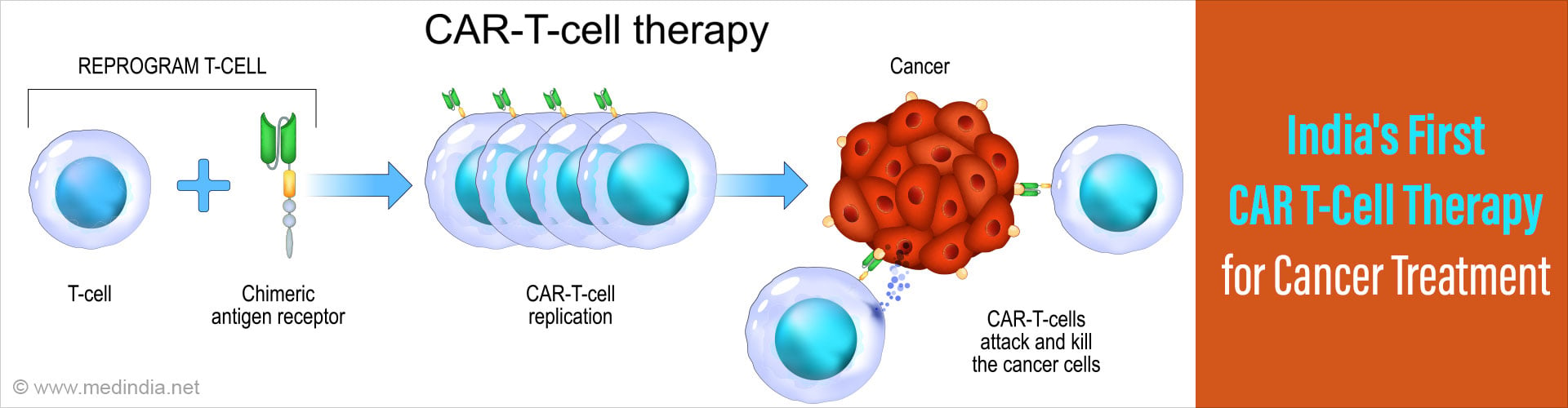
What is CAR T-cell Therapy?
At that time, CAR T-cell therapies were garnering attention worldwide for their potential to revolutionize cancer treatment. These innovative therapies involve modifying a patient's T cells in a laboratory to target and destroy cancer cells. However, the high cost and potential side effects associated with existing CAR T-cell therapies raised concerns about their accessibility and affordability, particularly in low- and middle-income countries like India. Undeterred by these challenges, Alka Dwivedi envisioned a different approach—one that would harness India's scientific prowess and address the unique needs of patients in her country. But she knew that she couldn't do it alone. Thus began a collaborative effort that would span continents and bring together experts from diverse fields.Did You Know?
CAR T-cell therapy costs around $400,000 in the US, while India's NexCAR19 is expected to be priced at approximately $50,000, making it more accessible.
Under the mentorship of NCI scientists, including Nirali Shah, M.D., the team delved into the nuances of CAR T-cell therapy, gaining invaluable insights into its design, development, and clinical application. The training provided at the NIH Clinical Center proved instrumental in shaping their approach to CAR T-cell therapy and laid the groundwork for their future endeavors.
Developing CAR T-cell Theraoy in India
Armed with newfound knowledge and a shared vision, Alka Dwivedi and her co-investigators returned to India with a mission—to develop an indigenous CAR T-cell therapy that could be produced locally, offered at an affordable cost, and tailored to meet the needs of Indian patients (1✔ ✔Trusted SourceDepartment of Biotechnology supported First CAR-T cell therapy conducted at ACTREC, Tata Hospital in Mumbai DBT/BIRAC-NBM Supported Phase I/II Clinical Trials
Go to source). Their journey was not without its challenges. Designing a CAR T-cell therapy from scratch required meticulous planning, innovative thinking, and unwavering dedication. But with each obstacle they encountered, the team remained steadfast in their pursuit of a solution.
Years of painstaking research and development culminated in the creation of actalycabtagene autoleucel (NexCAR19)—India's first approved CAR-T cell therapy. This groundbreaking achievement was made possible through a combination of scientific expertise, collaborative synergy, and unwavering determination.
In October 2023, the Central Drugs Standard Control Organization (CDSCO)—India's regulatory authority—approved NexCAR19, marking a historic milestone in the country's fight against cancer. The approval was based on the results of two clinical trials conducted in India, which demonstrated the therapy's efficacy and safety in patients with advanced lymphoma or leukemia.
According to trial data presented at the American Society of Hematology meeting, NexCAR19 showed promising results, with a significant proportion of patients experiencing a notable decrease in the extent of their cancer. Importantly, the therapy was well-tolerated, with minimal side effects reported.
New Era in Cancer Treatment in India
The approval of NexCAR19 heralds a new era in cancer treatment in India, offering hope to thousands of patients grappling with this devastating disease. But beyond its immediate impact, NexCAR19 represents a triumph of scientific innovation, collaborative partnership, and unwavering perseverance. Dr. Alka Dwivedi, now a trailblazer in the field of CAR T-cell therapy, continues her journey at NCI's Center for Cancer Research, where she is furthering her research and contributing to the advancement of cancer care. As India grapples with a growing burden of cancer, initiatives like NexCAR19 underscore the importance of homegrown solutions tailored to local needs. By harnessing the power of innovation, collaboration, and determination, we can overcome even the most daunting challenges and pave the way for a brighter, cancer-free future.Reference:
- Department of Biotechnology supported First CAR-T cell therapy conducted at ACTREC, Tata Hospital in Mumbai DBT/BIRAC-NBM Supported Phase I/II Clinical Trials - (https://pib.gov.in/PressReleasePage.aspx?PRID=1725254)
Source-Medindia