The United States Food and Drug Administration has approved the use of midostaurin drug along with chemotherapy for acute myeloid leukemia treatment.
Highlights
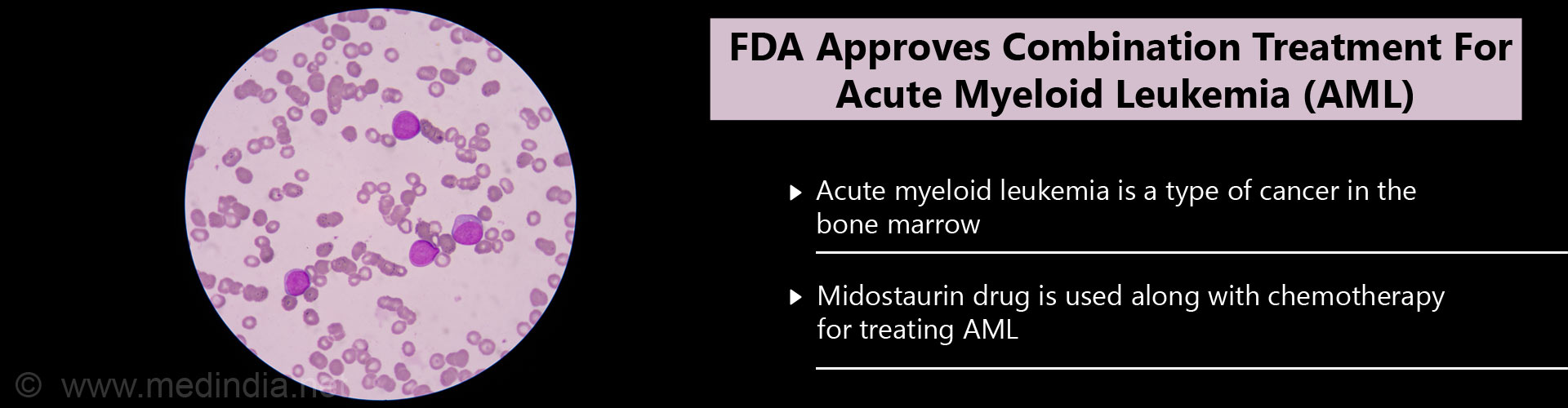
- Acute myeloid leukemia is a cancer of the blood and the bone marrow.
- FDA has approved the use of Midostaurin drug along with chemotherapy for treating acute myeloid leukemia.
- Patients who took a combination of midostaurin drug along with chemotherapy lived longer when compared to those who took chemotherapy alone.
The FDA approval for the drug was granted to Novartis Pharmaceuticals Corporation, while the approval for LeukoStrat CDx FLT3 Mutation Assay was granted to Invivoscribe Technologies.
“The ability to detect the gene mutation with a diagnostic test means doctors can identify specific patients who may benefit from this treatment.”
Studying the Efficacy and Safety of Midostaurin Drug
The efficacy and the safety of Midostaurin drug for patients with acute myeloid leukemia were studied by conducting a randomized trial on 717 patients who were not previously treated for acute myeloid leukemia.
The findings of the trial revealed that patients who received a combination treatment of midostaurin along with chemotherapy were found to live longer than those who received only chemotherapy.
The patients who received the combination of Midostaurin along with chemotherapy in the trial went longer without complications of the disease when compared to the patients who received chemotherapy alone.
Midostaurin (Rydapt) is a kinase inhibitor drug which works by blocking several enzymes that could promote cell growth.
The detection of the FLT3 mutation in blood or bone marrow samples, using the LeukoStrat CDx FLT3 Mutation Assay, may help the patient to be eligible for treatment in combination with chemotherapy.
Common side effects of Midostaurin drug include
- low levels of white blood cells with fever
- nausea
- mucositis (inflammation of the mucous membrane)
- headache
- vomiting
- musculoskeletal pain
- high blood sugar levels
- upper respiratory tract infections
- Midostaurin drug should not be used in patients who are allergic to the drug
- Women who are pregnant or breastfeeding must not take the drug as it could harm the fetus
- Patients with symptoms of lung damage should not use the drug due to pulmonary toxicity
It is a rapidly progressing cancer which is formed in the bone marrow and leads to an elevated white blood cell count in the bloodstream.
The National Cancer Institute estimated that around 19,930 people were diagnosed with AML in 2016. And around 10,430 people are projected to die due to the disease.
Facts on Acute Myeloid Leukemia (AML)
- Acute myeloid leukemia is the second most common type of cancer in children
- Acute myeloid leukemia (AML) is common among people who are exposed to large amounts of radiation and chemicals
- The five-year survival rate for acute myeloid leukemia ranges from 65-75%
- Around 43,000 people in the United States are affected with leukemia every year
- Smoking, genetic disorders and certain blood disorders like myelodysplatic diseases (chronic bone marrow diseases) are risk factors of acute myeloid leukemia
- FDA approves new combination treatment for acute myeloid leukemia - (https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm555778.htm)
- Acute Myeloid Leukemia (AML) - (https://www.stjude.org/disease/acute-myeloid-leukemia.html)
- Leukemia Facts - (https://www.mdanderson.org/cancer-types/leukemia/leukemia-facts.html)