Subtype of neuron rapidly increases or decreases hunger via fast-acting glutamate neurotransmitter.
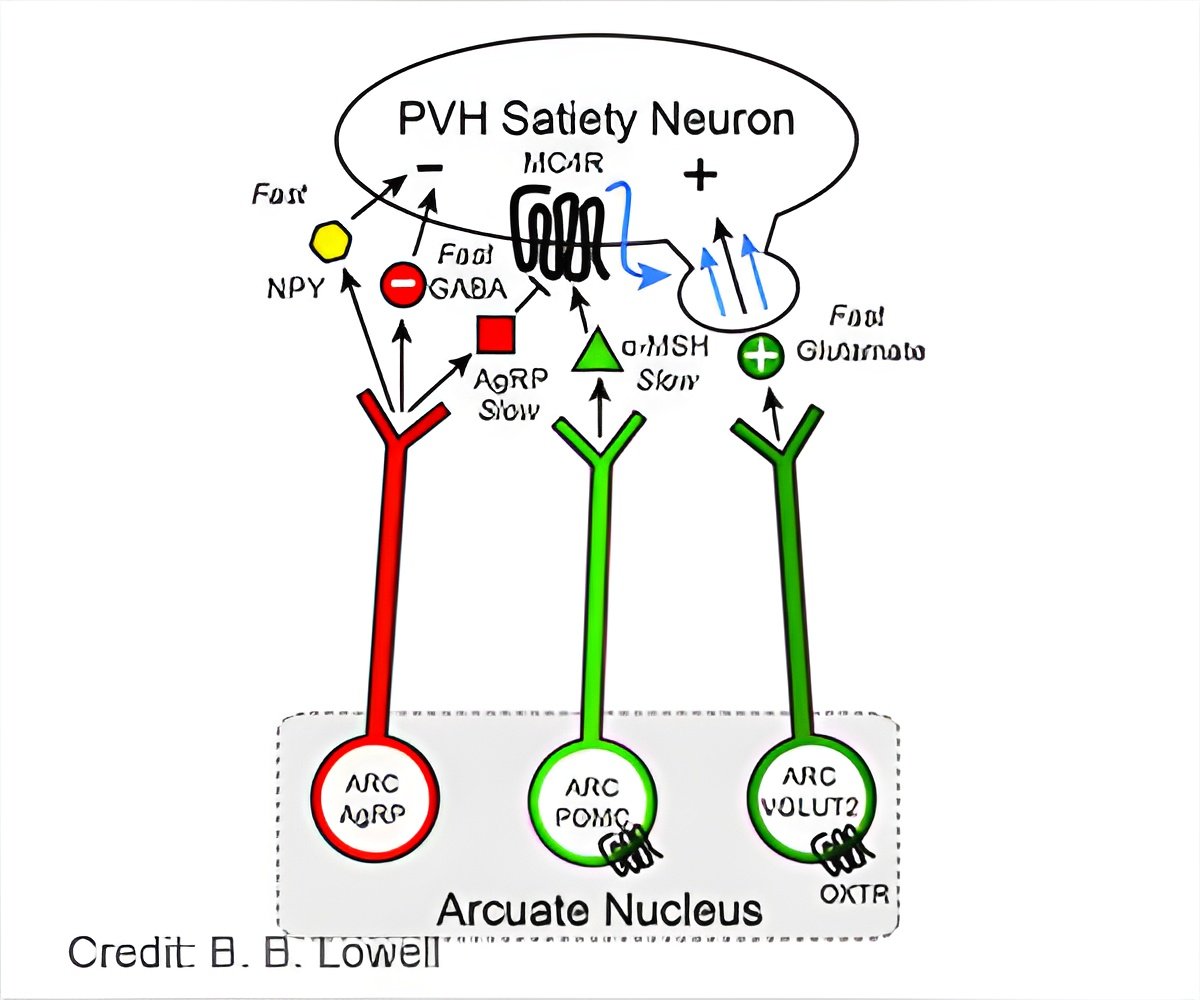
- Hunger and satiety are controlled by two kinds of neurons, Agouti-related protein (AgRP) neurons and pro-opiomelanocortin (POMC) neurons.
- Lack of POMC neurons caused obesity in mice.
- The POMC neurons act over the course of many hours, which suggested that unknown neural component of the satiety system was missing from the current models.
- This led to the discovery of a third subset of neurons, Vglut2, which rapidly suppresses feeding behavior. Previously unknown neural circuitry that plays a role in promoting satiety, the feeling of having had enough to eat, has been identified by researchers at the Beth Israel Deaconess Medical Center (BIDMC). The discovery revises the current models for homeostatic control (the mechanisms by which the brain maintains the body's status quo) of feeding behavior.
- Agouti-related protein (AgRP) neurons- These neurons drive hunger; stimulating these nerves triggers eating within minutes.
- pro-opiomelanocortin (POMC) neurons- The opposing group of neurons, POMC, has been shown to promote satiety.
"Current models lack a rapidly-acting satiety mechanism analogous to the hunger-promoting neurons," said corresponding author Bradford B. Lowell, MD, PhD, a professor of medicine in the Division of Endocrinology, Diabetes and Metabolism at BIDMC. "This component we identified provides the full 'yang' to the hunger-promoting neurons' 'yin.'"
Hunger and Satiety Neurons-
Researchers have long known that hunger is regulated by two kinds of neurons:
However, in recent years, new technology has allowed scientists to selectively manipulate neurons in living animals, demonstrating specific neurons' direct effect on behavior.
About five years ago, this type of research revealed that POMC neurons act over the course of many hours -- not minutes like their AgRP counterparts.
The team was looking for neurons that would suppress eating quickly, they narrowed their search for ones that release the fast-acting, excitatory neurotransmitter called glutamate.
In the current paper, Lowell and colleagues investigated a third subset of neurons, Vglut2, the function of which was previously unknown.
Role of Vglut2 neurons
In a series of experiments, Lowell and colleagues used optogenetics (the use of light to activate genetically-modified neurons) and chemogenetics (the use of molecules to activate targeted neurons) to manipulate specific neurons to determine their roles in feeding behavior.
First, the team demonstrated that, unlike POMC neurons, Vglut2 neurons could in fact rapidly suppress feeding behavior in mice.
When the researchers chemically stimulated the neurons in question, it markedly decreased eating. When they inhibited the Vglut2 neurons, eating increased.
They also found that all three types of neurons -- the hunger promoting AgRP neurons and the two satiety promoting neurons, Vglut2 neurons and POMC neurons -- interact in the same circuit.
All three neurons converge downstream in a site in the hypothalamus known to suppress hunger.
AgRP neurons inhibit activity in this downstream site, causing hunger, while the rapidly-acting Vglut2 neurons excite it, suppressing hunger.
The third member of this circuit, POMC neurons, indirectly decrease hunger by releasing a hormone that bolsters Vglut2 neurons' ability to suppress hunger.
"We think this is very important because it may be one explanation why individuals lacking POMC genes become extremely obese, even if those neurons by themselves do not directly promote satiety," said Fenselau.
"Without POMC, the Vglut2 neurons are ineffective in stimulating the downstream hypothalamic site. You need both POMC neurons and Vglut2 neurons for the satiety mechanism to work properly." Fenselau added.
The team then sequenced the genes expressed by this subset of neurons and found they possess receptors for the hormone oxytocin.
Known to play a role in social bonding, oxytocin is linked to feelings of contentment and is already being investigated for its potential as a satiety-inducing weight loss medication. "Our findings could definitely explain why oxytocin is effective in satiety," said Fenselau.
To follow up, the scientists will further investigate the interactions among the three types of neurons and the role they play in obesity.
Source-Medindia