PEARL uses cfDNA to detect preeclampsia early, improving pregnancy outcomes and reducing complications for mothers and babies.
- PEARL uses cfDNA analysis for early, non-invasive preeclampsia detection
- It simplifies screening by requiring only cell-free DNA (cfDNA), blood pressure, and Body Mass Index (BMI)
- PEARL currently depends on advanced sequencing technology, limiting accessibility in low-income countries
Preeclampsia risk prediction from prenatal cell-free DNA screening
Go to source). In severe cases, it may lead to premature birth. Early detection of preeclampsia is important. However, current methods have limitations in accuracy, hindering early diagnosis.
Preeclampsia causes over 70,000 maternal deaths and 500,000 fetal deaths worldwide every year. #prenatalhealth #hypertension #preeclampsia #medindia’
Early Detection and the Role of Placenta
Early detection of preeclampsia can help doctors take preventive measures before it causes any other health issues. However, current prediction methods rely on checking clinical factors like blood pressure and body mass index or analyzing specific biomarkers in the blood. These methods are not always reliable and accurate.The placenta plays an important role in developing preeclampsia. The placenta cannot be examined directly during pregnancy. So, using a non-invasive approach, the cell-free DNA (cfDNA) released from the placenta into the mother's bloodstream can be analyzed. This process starts early in pregnancy and has important information about the baby and the placenta’s condition.
Using cfDNA to Predict Preeclampsia
Researchers have developed a new method called PEARL (Preeclampsia Early Assessment of Risk from Liquid Biopsy) to assess preeclampsia risk. This method uses cfDNA from standard prenatal screening tests to analyze how DNA fragments are arranged. Since cfDNA is modified by the placenta and maternal blood vessels, examining its structure can help detect early signs of preeclampsia.PEARL uses cfDNA sequencing data, collected for routine prenatal tests like checking for chromosomal disorders in the baby. By analyzing the structure and origin of cfDNA fragments from the placenta and maternal blood vessels, PEARL can detect signs of tissue damage linked to preeclampsia months before symptoms appear.
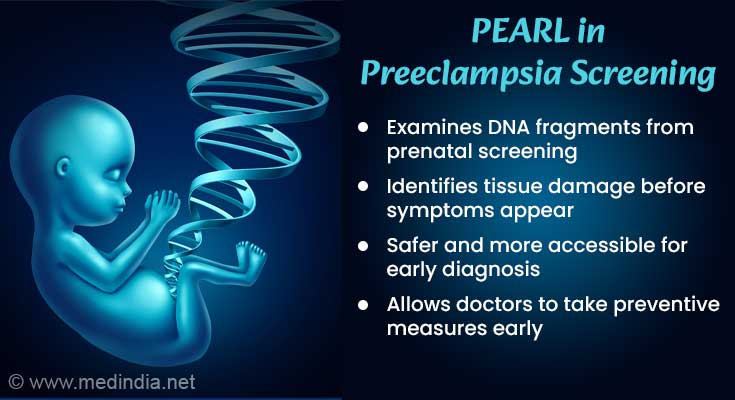
Why PEARL is Better Than Current Methods
Current screening methods like Fetal Medicine Foundation’s first-trimester test, analyze clinical and laboratory factors like racial background, placental growth factor levels, and ultrasound findings. Though effective, these methods are complex and may not be accurate for all populations. While PEARL requires two additional factors—blood pressure and BMI to make it simpler and more accessible.Early detection of preeclampsia allows doctors to prescribe low-dose aspirin or closely monitor blood pressure. Studies show that managing blood pressure effectively can reduce the risk of severe complications, including premature birth and high blood pressure. As this non-invasive detection method is safe, early screening can help doctors decide who needs extra monitoring or treatment.
Challenges and Future Directions
PEARL was tested on a wide range of pregnancies, including those with pre-existing conditions like diabetes and high blood pressure. This makes the findings more effective in real-world cases. It is important to conduct larger studies to validate the accuracy of this method and how it can be used in routine prenatal check-ups. Conclusion: A Step Forward in Preeclampsia Prediction.Although PEARL is a promising tool, it currently relies on sequencing technology available mostly in high-income countries. Transporting blood samples to specialized labs for analysis may not be feasible in low- and middle-income countries. Future research is needed to make this test more widely available and affordable.
PEARL offers a cost-effective and scalable solution. With further research, this method could become a standard tool for early preeclampsia screening, improving pregnancy outcomes and reducing complications for mothers and babies.
Reference:
- Preeclampsia risk prediction from prenatal cell-free DNA screening - (https://www.nature.com/articles/s41591-025-03509-w)
Source-Medindia