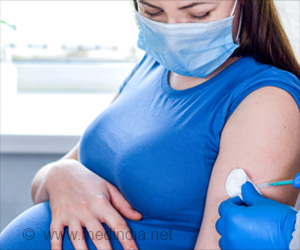
European Medicines Agency has granted marketing authorization for Abrysvo, a vaccine to protect against disease caused by the respiratory syncytial virus(RSV).
- Respiratory Syncytial Virus (RSV) can cause serious consequences for children and older adults
- It is the leading cause of pediatric hospitalization and fatal respiratory distress in Europe
- Regulators have given nod to a bivalent vaccine against RSV-A and B strains based on clinical trial results
Respiratory Syncytial Virus (RSV): It's More Than Just a Cold
RSV is a common respiratory viral infection presenting mild, cold-like symptoms in children and adults. Most people recover within two weeks but it can cause serious health consequences such as bronchiolitis (inflammation of the small airways in the lung) and pneumonia (infection of the lungs), especially for infants and older adults (1✔ ✔Trusted SourceRespiratory syncytial virus: from pathogenesis to potential therapeutic strategies
Go to source).
RSV being a burden to nations, the quest for vaccines started many years before. The development began in the 1960s with an unsuccessful formalin-inactivated RSV (FI-RSV) vaccine that induced a fatal response. This response to natural RSV infection has been referred to as vaccine-associated enhanced respiratory disease (ERD).
The concerns over this response hindered the development of alternative RSV vaccines for many years. In recent years, increased understanding of the RSV and associated technological advances have resulted in the entry of multiple vaccine candidates into clinical trials.
Europe Approves New Vaccine to Protect Babies and Adults Against RSV
One among them is Abrysvo. It is a bivalent vaccine that gives protection against RSV A and B strains. The vaccine contains proteins essential for RSV to infect the body and are also the main targets of the antibodies generated to fight the infection. Abrysvo was evaluated under European Medicines Agency (EMA)’s assessment mechanism before entering the market.Generally, the vaccine works by generating specific antibodies and T cells (immune system cells) that help prevent RSV infection. In the case of pregnant women, the antibodies cross the placenta to provide infants with protection up to 6 months after birth.
EMA’s human medicines committee (CHMP) assessed the working efficacy of the RSV vaccine based on data from two randomized, placebo-controlled, pivotal studies.
Efficacy and Safety of a Bivalent RSV Prefusion F Vaccine in Older Adults
Go to source) .
In the other study, 18,488 adults aged 60 years and older were administered the vaccine. The results of the study showed a reduction of RSV-associated lower respiratory tract illness with 3 or more symptoms.
The opinion adopted by the CHMP is the beginning step on Abrysvo’s path to patient access. The opinion will now be sent to the European Commission to decide its authorization for EU-wide marketing (3✔ ✔Trusted Source
Meeting highlights from the Committee for Medicinal Products for Human Use (CHMP) 17-20 July 2023
Go to source).
Once a marketing authorization has been granted, decisions about price and reimbursement will take place at the level of each Member State, considering the potential role/use of this medicine in the context of the national health system of that country.
References:
- Respiratory syncytial virus: from pathogenesis to potential therapeutic strategies - (https://www.ijbs.com/v17p4073.htm)
- Efficacy and Safety of a Bivalent RSV Prefusion F Vaccine in Older Adults - (https://www.nejm.org/doi/10.1056/NEJMoa2213836)
- Meeting highlights from the Committee for Medicinal Products for Human Use (CHMP) 17-20 July 2023 - (https://www.ema.europa.eu/en/news/meeting-highlights-committee-medicinal-products-human-use-chmp-17-20-july-2023)
Source-Medindia