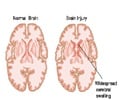
Traumatic brain injury outcome can now be predicted with an easy to administer, safe and inexpensive blood test.
- Higher levels of the protein biomarkers UCH-L1 and GFAP are linked to severe damage and death in patients with traumatic brain injury.
- The test will enable medical professionals in determining the severity of a brain injury.
- Clinicians will also be able to advise families on how to care best for their loved ones who have suffered a brain injury and what to anticipate for their recovery.
Importance of Protein Biomarker Blood Test in Modern Trauma Care
“Early and accurate prediction of TBI outcomes will help clinicians gauge how severe a brain injury is and inform how best to counsel family members about care for their loved ones with brain injury and what to expect with regards to their recovery. It will also help researchers more precisely target promising TBI therapeutics to the right TBI patients,” said Korley.The usage of GFAP and UCH-L1 was approved by the U.S. Food and Drug Administration in 2018 to assist doctors in determining whether to obtain CT scans for mild traumatic brain injury.
The i-STAT Alinity and the ARCHITECT, both from Abbott Laboratories, were used to measure the proteins. The Glasgow Outcome Scale-Extended, a measure that rates the functional state of TBI patients, was used to compare the results to assessments carried out six months following the injury.
“Modern trauma care can result in good outcomes in what we had once believed were non-survivable injuries. These blood tests are both diagnostic and prognostic, as well as easy to administer, safe and inexpensive,” said co-senior author Geoffrey Manley, M.D., PhD, professor and vice chair of neurosurgery at UCSF.
Further research into the Role of New Biomarkers in Mild Brain Injury
Despite the promising findings for predicting bad outcomes in moderate and severe TBI, researchers suggest further research to fully understand the role of these biomarkers in mild TBI cases.“As a next step, the TRACK-TBI team is planning a clinical trial that will examine the efficacy of promising therapeutic agents that may help traumatic brain injury patients recover quickly. As part of this clinical trial, these biomarkers will be used as an objective method for selecting the right patients to enrol in this trial. We will also use these biomarkers to monitor individual patient response to these promising therapeutics,” said Korley.