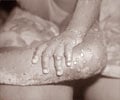
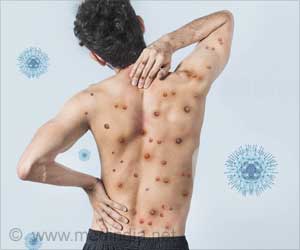
Whether monkeypox patients receiving tecovirimat heal more quickly than those receiving a placebo is being studied by researchers.
- Currently, there are no approved treatments for human monkeypox
- Tecovirimat, approved by the US FDA to treat smallpox, is being studied to evaluate its safety and efficacy to treat monkeypox
Worldwide Monkeypox Virus Outbreak
Monkeypox began to spread worldwide in the spring of 2022, with more than 56,000 cases reported in 103 nations, including more than 21,000 cases in the United States. Since 1958, when monkeypox was first discovered in endemic nations, there have been more illnesses each year. Person-to-person transmission has increased during the current pandemic. It is thought that close touch during sexual activity has a significant impact on this outbreak. Although men who have sex with other men have been reported to have most cases so far, women and children have also contracted the disease. There are no approved treatments for human monkeypox now.Can Tecovirimat be Used to Treat Monkeypox?
There will be more than 500 adults with monkeypox virus infection enrolled in this multi-center experiment. Importantly, this trial will include participants with severe illness and those at high risk of developing severe illness, such as women who are pregnant or nursing, kids, people who have underlying immune deficiencies and people who have active inflammatory skin conditions and will receive open-label tecovirimat. Tecovirimat or placebo will be administered orally for 14 days to study participants with symptomatic monkeypox virus infection who do not meet the requirements for the open-label cohort.Participants were randomized in the double-blinded group of the study and later developed the severe disease, as well as those who report ongoing, excruciating pain from monkeypox virus infection, will be given the choice to switch to open-label tecovirimat.
To find out if those getting tecovirimat heal more quickly than those receiving a placebo, all participants in STOMP will be monitored for at least eight weeks by a combination of virtual and in-person visits, daily self-reports, and other methods. Critical information regarding the safe administration of tecovirimat to children, pregnant women, and nursing mothers will also be made available by STOMP.
Candidates must have either a presumed or confirmed case of monkeypox (testing positive within seven days) and have shown symptoms for at least 13 days. The study will provide testing. If their study-provided test is positive, participants with presumptive monkeypox virus infection who have not yet been tested are eligible to enrol. Additionally, participants must have proctitis, a mouth lesion, or at least one active skin lesion that has not yet been scabbed (inflammation in the lining of the rectum).
Source-Medindia