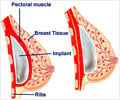
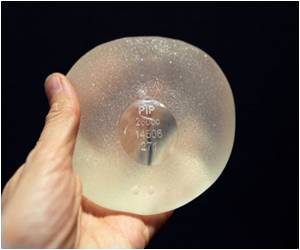
Rupture rates on allegedly faulty French-made breast implants are seven times higher than previously thought, reveals data.

The firm's figures suggest one in 14 implants made by French manufacturer Poly Implant Prothese (PIP) had ruptured since 2006 -- about seven percent.
If replicated across the industry, this would represent a far higher risk than is currently estimated by Britain's Medicines and Healthcare products Regulatory Agency (MHRA), which puts the rupture rate at one percent.
The French regulatory authority, AFSSAPS, meanwhile suggests a failure rate, including rupture, of about five percent in France, MHRA said. Health officials have advised 30,000 French women with PIP implants to have them removed.
"We believe -- and it needs rechecking -- that the rupture rate is actually higher at about seven percent," a source at Transform told the Sunday Telegraph.
The company was unavailable for comment on Sunday, while a spokesman for the Department of Health could not confirm the figures.
Advertisement
On Saturday, Lansley said he was "concerned" and "unhappy" about inconsistencies in the data surrounding the risks of PIP implants, after a private provider came forward with new information.
Advertisement
The review, led by the medical director of the National Health Service (NHS), Bruce Keogh, will also look at whether improvements are needed in the regulation of cosmetic surgery in Britain. It will report back next week.
The now-bankrupt PIP was shut down and its products banned in 2010 after it was revealed to have been using non-authorised silicone gel that caused abnormally high rupture rates in its implants.
Source-AFP