Researchers are working together to enhance the drug delivery system that gained prominence through the COVID-19 vaccines.
Correlating the Structure and Gene Silencing Activity of Oligonucleotide-Loaded Lipid Nanoparticles Using Small-Angle X-ray Scattering
Go to source). The first drug to use LNPs was approved in 2018, but the delivery method rose to global prominence with the Pfizer and Moderna mRNA COVID-19 vaccines.
‘The widespread exploration of lipid nanoparticles (LNPs) as a delivery system extends to vaccines for various infectious diseases and therapeutic vaccines for cancer. #drugdelivery #nanotechnology #nanoparticles’





“It’s quite a smart system, because if you just deliver the RNA itself to the human body, the RNA is degraded by nucleases and cannot easily cross the cell membrane due to its size and charge, but the LNPs deliver it safely into the cell,” explained co-lead author Chun-Wan Yen, a senior Principal Scientist in Genentech’s Small Molecule Pharmaceutical Sciences group. The viability of these new applications will be dependent on how well the lipid envelopes fuse with target cells, how stable the drug-LNP formulations are in storage (so that they have a long shelf-life), and how stable they are in the body (so they can confer prolonged drug activity).
All these properties are controlled by the mixture of molecules used to create the LNP, and the resulting 3D structure of the particle. The team under Yen and fellow co-leads Greg Hura and Michal Hammel, both Berkeley Lab biophysicists, has been studying how to tune the structure of LNPs for desired properties for several years.
High-Throughput Workflow for Rapid Production and Characterization of LNPs
Their latest paper, published recently in ACS Nano, documents how a high-throughput workflow allows them to produce and characterize LNPs at record speed. The study also includes the first-ever demonstration of how LNP structure correlates with the activity of its contents, which for this investigation was an anti-sense oligonucleotide (ASO). ASOs are small snippets of RNA or DNA base pairs that block gene expression by binding to strands of mRNA and preventing them from being translated into proteins. ASOs are a great way to treat diseases caused by faulty proteins or the over-abundance of a protein. But, like mRNA, they are susceptible to roving nucleases – enzymes that degrade RNA and DNA – and cells do not readily uptake them.The scientists discovered that ASO-carrying LNPs with neatly ordered, closely-packed internal structures led to better silencing of a faulty gene in human neurons that is associated with a degenerative disease, compared with LNPs that had a more disordered structure. The findings were from cell-based studies, not from animal studies, so there is still more work ahead, but the team are excited to build upon these insights using the complementary tools of each institution.
“We generate the LNPs in high-throughput and Greg and Michal’s team can offer the high-throughput analysis,” said Yen. “If you check about the publication nowadays, they typically just do one or two formulations, but for us it's different. We can generate large datasets, and I think that's the reason why we can have this very unique and cool finding.”
Advertisement
How to Build a Lipid Nanoparticle
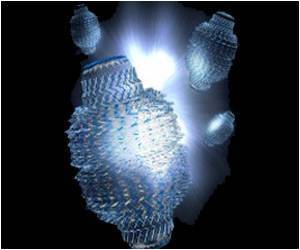
The structures of LNPs are affected by how you mix them, what you mix together, and in what order. LNPs have four ingredients – ionizable lipids, helper phospholipids, cholesterol, and polyethylene glycol-lipids (PEG-lipids) – and each ingredient has different forms. Plus, they can be combined in different ratios, leading to an exponential number of possible formulas. Further complicating matters, the LNPs change with time. A formulation that begins as a neat, close-packed sphere will morph into a more disordered structure eventually.Scientists at Genentech developed a robot-driven workflow that can generate hundreds of LNP formulations in just a few hours. Samples of each formulation are then brought to Berkeley Lab to perform small-angle X-ray scattering (SAXS) at the Advanced Light Source, a circular particle accelerator that creates X-ray beams of different energies.
Advertisement
Additionally, the Genentech team uses an accelerated process to study how LNPs affect gene expression in their target cells. By combining all these expedited techniques, the entire collaboration is able to screen potential LNPs at an unprecedented rate.
Yen plans to continue using the SAXS beamline to study small details, like how a 1% change in ingredient concentration or using a new machine during production can affect LNP’s cellular activity, as well as big questions, such as whether LNPs behave differently if they are carrying other cargo types and how they interact with different target cells.
“We know that mRNA LNPs work, but there is still a huge knowledge gap,” Yen said. “That's why I feel like our paper is a pioneer in this field and hopefully we can also generate more data and understanding for the future applications.”
Reference:
- Correlating the Structure and Gene Silencing Activity of Oligonucleotide-Loaded Lipid Nanoparticles Using Small-Angle X-ray Scattering - (https://pubs.acs.org/doi/10.1021/acsnano.3c01186)