When fibroid cells in culture are subjected to mechanical strain, various signaling pathways are triggered, opening new avenues for non-hormonal therapies.
Uterine fibroid cell cytoskeletal organization is affected by altered G protein-coupled estrogen receptor-1 and phosphatidylinositol 3-kinase signaling
Go to source).
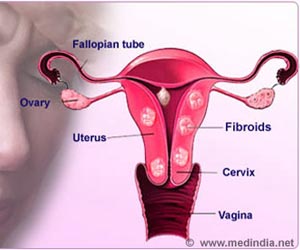
How Do Painful Uterine Fibroids Grow?
Nearly 8 in 10 women develop fibroids, noncancerous tumors that develop in the uterus during child-bearing years. They can be extremely painful, cause extensive bleeding, and lead to infertility.‘Fibroids affect over 4 out of 5 women in their lifetime, and they cost the US economy $9 billion annually. #fibroids #uterinecancer #nonhormonaltherapy #womenhealth’





“That’s important for identifying therapeutic targets because we want to target the tumor without affecting the surrounding tissue,” said Stacey Schutte, an assistant professor of biomedical engineering at UC’s College of Engineering and Applied Science.Treating fibroids is often invasive and expensive, costing patients and their insurers billions of dollars each year, according to the National Institutes of Health. Treatments can often lead to infertility as well, Schutte said.
“One in nine women will have a hysterectomy in their lifetime. And one-third to one-half of those are [because of] uterine fibroids,” Schutte said. “It usually isn’t life-threatening, but the pain can be immense,” she said. “Contractions push the tumors into the muscle tissue.”
During each menstrual cycle, the body releases estrogen and progesterone, which causes the tissue lining inside the uterus to thicken in anticipation of possible pregnancy. These hormones also help fibroids grow.
But Schutte said cells likewise can react to physical strain — like a defense mechanism to protect the cells.
Advertisement
“We have a flexible tension device. We grew cells on plates with an elastic bottom. Then we used a vacuum to pull and stretch it,” Schutte said. “It stretches cells in a single direction.” “We found that fibroid cells were more sensitive to strain,” said study lead author Rachel Warwar, MD, in UC’s College of Medicine.
“The more we are able to mimic the environment of these cells in the uterus, the more we will understand the pathology of these cells and can then work to target anomalous pathways in fibroid cells,” she said.
Common noninvasive treatments target hormones responsible for fibroid growth. “We are looking for nonhormonal treatments for fibroids,” said study coauthor Andreja Moset Zupan, a research associate in Schutte’s biomedical engineering lab.
“It’s another option we could use to preserve the fertility of women who still want to get pregnant,” she said. Once researchers understand the cell pathology, Warwar said, they can study fibroids using 3D simulations and modeling, which could help them further understand how fibroids develop and the best ways to treat them.
Schutte said the next step is to create more complex tissue models to mimic tumor growth to learn ways to inhibit it. “It makes me really happy to think we can find a target.”
Reference:
- Uterine fibroid cell cytoskeletal organization is affected by altered G protein-coupled estrogen receptor-1 and phosphatidylinositol 3-kinase signaling - (https://www.fertstertscience.org/article/S2666-335X(23)00055-1/fulltext)