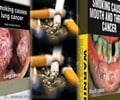
For tobacco products, when the U.S. Food and Drug Administration (FDA) imposes new graphic warning labels, they can survive a First Amendment challenge if they depict health consequences and their effectiveness is supported by adequate scientific evidence.

Graphic tobacco warning labels—which combine images with health warnings—are a widely used tool for reducing tobacco use in other countries, but the tobacco industry argues they are unconstitutional in the United States.
In an analysis of legal and scientific issues for graphic warning labels published in the American Journal of Preventive Medicine, John Kraemer, JD, MPH, outlines how the courts will likely analyze graphic warnings and identifies what health evidence must be presented to survive a legal challenge. Kraemer is an assistant professor of health systems administration at Georgetown University School of Nursing & Health Studies and member of the O'Neill Institute for National and Global Health Law.
Despite the fact that smoking kills 443,00 Americans each year, Kraemer says, "The U.S. has some of the weakest tobacco warning labels in the world, and they haven't been updated in almost 30 years."
In 2009, Congress passed the Family Smoking Prevention and Control Act requiring graphic warning labels on tobacco products, giving the FDA authority to specify the images and text that must be included. The FDA issued nine graphic warnings in June 2011, but withdrew them after two federal appeals courts came to opposite conclusions about their constitutionality.
Though ambiguity exists over what constitutional standard would be applied in a legal challenge to the labels, Kraemer argues that it is possible for the FDA to meet the two most likely standards —rational basis review and intermediate review—with the right scientific evidence.
Advertisement
The second possibility is intermediate review, which requires a stronger governmental interest and greater certainty that warning labels would be effective.
Advertisement
He adds that the FDA must also take care to avoid images that could be interpreted as opinions instead of facts or which do not show a negative health consequence of smoking, such as an image previously adopted by the FDA, which depicted a man with a no-smoking sign on his shirt.
Source-Eurekalert