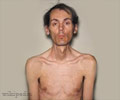
In a new potential therapy a natural human protein significantly decreased muscle damage and improved muscle function in mice with muscular dystrophy, according to a paper.

"This is all aimed at getting a therapy that will meaningfully improve the condition of patients," said Justin Fallon, professor of neuroscience at Brown University and the senior author of the paper. "This is an important step along that path."
This fall, the startup company Tivorsan Pharmaceuticals licensed rights from Brown to the key protein, biglycan, hoping to bring the potential therapy through clinical trials.
Biglycan restores the muscle-strengthening presence of a protein called utrophin, which is normally prevalent only in very young children. Utrophin still exists in adults, but in fewer places and not where it can help muscular dystrophy sufferers who cannot produce dystrophin, which keeps adult muscles strong.
Encouraging experiments
In experiments described in the paper, Fallon's team showed that biglycan delivered to the bloodstream draws utrophin to the cellular membranes of muscle cells. Much as utrophin does when it is present in fetuses, infants and toddlers, the protein works to help the cells build and retain their strength.
Advertisement
The team also subjected mouse muscles to a standardized stress test where they are simultaneously stretched and caused to contract. The test ultimately weakens even healthy muscle, but in the tests conducted by researchers and co-authors from the University of Pennsylvania, the muscles of muscular dystrophy mice treated with biglycan lost their strength 30 percent more slowly than similar mice who were untreated.
Fallon said the effects of treatment with biglycan lasted through months of testing. Several basic tests for side effects during that time frame, such as kidney and liver function, did not indicate any harm from the therapy.
With the efficacy of biglycan apparent in the mouse model of Duchenne Muscular Dystrophy, Fallon is eager to see if it can improve the lives of thousands of children.
"The next big step is testing in humans," Fallon said.
Source-Eurekalert