Hydronidone is an investigational drug targeting liver fibrosis in chronic hepatitis B patients.
Hydronidone for the Treatment of Liver Fibrosis Associated with Chronic Hepatitis B: Protocol for a Phase 3 Randomized Trial
Go to source). In our Phase 2 trial, the addition of hydronidone to entecavir in patients with chronic hepatitis B (CHB)-related liver fibrosis led to a significant reversal of fibrosis. To further assess the efficacy of hydronidone at a 270 mg/day dosage in treating CHB-associated liver fibrosis, we initiated this Phase 3 trial.
‘Exciting development in #HepatitisB treatment! A new #clinicaltrial is investigating hydronidone's potential to combat #liverfibrosis while ensuring safety in #hepatitis patients. #HBV. #worldliverday’





Hydronidone vs. Placebo for Liver Fibrosis
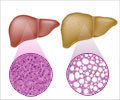
This is a 52-week, randomized (1:1), double-blind, placebo-controlled, multicenter, entecavir-based Phase 3 clinical study conducted at 44 study centers across China. Adult patients aged 18 to 65 years with significant liver fibrosis (defined as an Ishak score ≥ 3 on liver biopsy) associated with CHB were included.Liver fibrosis is a wound-healing response to chronic liver injury, characterized by the excessive accumulation of extracellular matrix proteins, including collagen. It occurs as a result of sustained inflammation and damage caused by various conditions such as chronic hepatitis B or C infection, alcohol abuse, nonalcoholic fatty liver disease (NAFLD), or autoimmune liver disorders.
Over time, the continuous scarring disrupts the normal liver architecture and impairs its function. If left untreated, liver fibrosis can progress to cirrhosis, liver failure, or hepatocellular carcinoma. While early-stage fibrosis may be reversible with appropriate treatment of the underlying cause, there are currently no approved therapies that specifically target the fibrotic process itself, highlighting a significant unmet need in liver disease management.
The primary endpoint of the trial is to demonstrate the efficacy of fibrosis reversal, defined as a decrease in the Ishak stage score of liver fibrosis by ≥1 after 52 weeks of treatment, compared to baseline.
This trial aimed to identify the antifibrotic effect and safety of hydronidone for CHB patients with significant liver fibrosis. It is anticipated that the findings of this study will further support the antifibrotic indication for this novel drug.
Advertisement
- Hydronidone for the Treatment of Liver Fibrosis Associated with Chronic Hepatitis B: Protocol for a Phase 3 Randomized Trial - (https://www.xiahepublishing.com/2310-8819/JCTH-2024-00472)
Source-Eurekalert