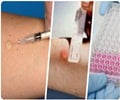
Indian Council of Medical Research (ICMR) launches a vaccine trial to prevent the occurrence of tuberculosis (TB) among close contacts of a TB patient.
‘ICMR is testing TB vaccine for protection from TB infection even when around an active TB patient. This vaccine trial is an essential step in prevention and in reducing the burden of the disease.
’
Read More..




This vaccine trial is an important step in prevention and decreasing the burden of this disease, said a release from ICMR. Read More..
"After a detailed landscape analysis of the available lead vaccine candidates, two potential vaccine candidates - VPM 1002 produced by Serum Institute of India in Pune and Mycobacterium Indicus Pranii (MIP) were shortlisted for taking forward through the phase III vaccine trial in healthy household contacts of sputum smear-positive TB patient," the ICMR said in a statement.
The study will test the safety and efficacy of these two vaccines by administering them in close TB contacts as opposed to a control group of close TB contacts who will not receive the vaccine. The study will enroll 12,000 healthy household contacts of sputum smear-positive TB cases that are at high risk of contracting the disease, from seven sites in six states - Delhi, Karnataka, Maharashtra, Orissa, Tamil Nadu, and Telangana.
Balram Bhargava, Secretary, Department of Health Research and Director General, ICMR said the clinical trials are needed in India to show that the vaccine is safe and effective and that it can provide protection to Indian populations where the disease is endemic.
Rohit Sarin, Director, National Institute of Tuberculosis and Respiratory Diseases (NITRD) said that this is the much-awaited trial and assured full support in timely completion of the trial.
Advertisement
Source-IANS