Many efforts have been made to develop therapies against the growth of blood vessels and 'starve' tumours, but they are not entirely effective.
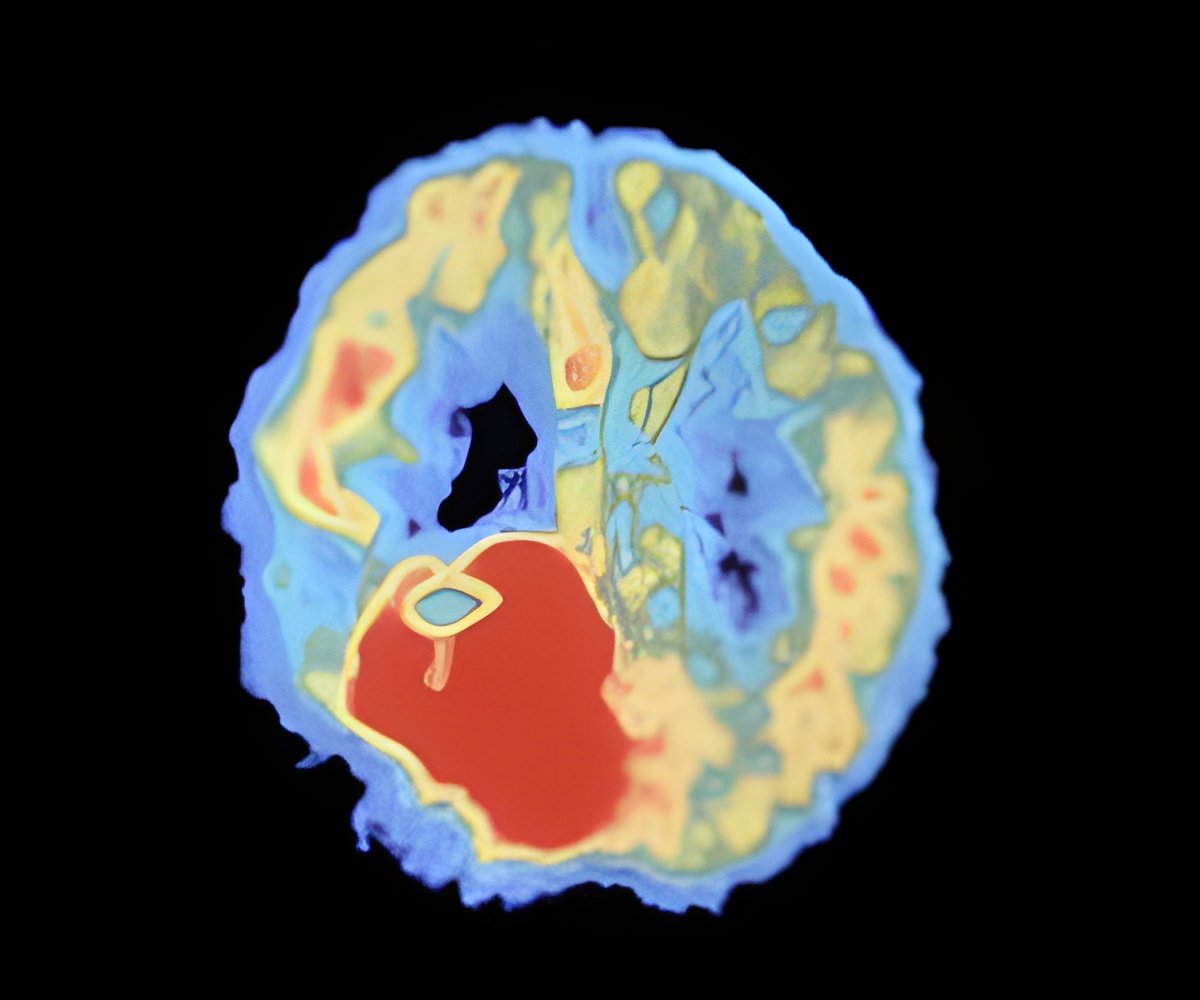
‘Improved imaging techniques that faithfully show the vessel architecture, structure, and the effects of treatments in a non-invasive way are therefore needed to inform the development of future clinical trials.’





"Many efforts have been made to develop therapies against the growth of blood vessels and therefore 'starve' tumours of their resources, but they are not entirely effective. Improved imaging techniques that faithfully show the vessel architecture, including their growth, structure and density, and the effects of treatments in a non-invasive way are therefore needed to inform the development of future clinical trials." In their study in mice, the team combined an MRI approach in vivo with ultramicroscopy of ex vivo whole brains cleared for imaging. The technique is based on T2*-weighted (T2*-w) MRI images, one of the basic pulse sequences in MRI, with high resolution to allow for substantially more detail than conventional T2*-w imaging. Pre- and post-contrast MR scans were performed to define the growth of vessels during glioma development in two different glioma models.
The team further mapped the development of vessels by dual-colour ultramicroscopy of whole, cleared brains. Using fluorescent labelling of microvessels, they collected complementary 3D MR and ultramicroscopy data sets (dubbed the 'MR-UM'), which could be compared side-by-side.
"MR-UM can be used as a platform for three-dimensional mapping of single vessels and detailed measurements of the growth of newly formed vessels over time," Dr. Breckwoldt explains.
"This provides a better understanding of the underlying mechanisms of existing treatment and could help identify novel targets for future drug development," adds Dr. Julia Bode, co-lead author from the German Cancer Research Centre.
Advertisement
The T2*-weighted imaging sequence and UM studies in ex vivo brains are at present only suitable for mapping tumour vessels in a preclinical setting. The team anticipates, however, that future studies using high-field clinical MR systems should enable possible translation of the MRI approach to the clinical arena. Furthermore, specimens taken for clinical diagnosis could be studied using ultramicroscopy, making the full MR-UM toolkit a potential player in a clinical setting.
Advertisement