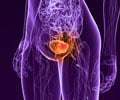
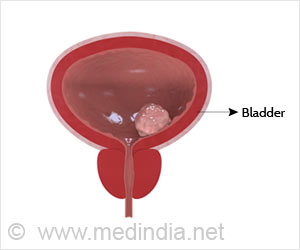
Is there a chance of staying cancer free after bladder cancer? Yes, immunotherapy increased this chance after surgery compared to patients who received a placebo.
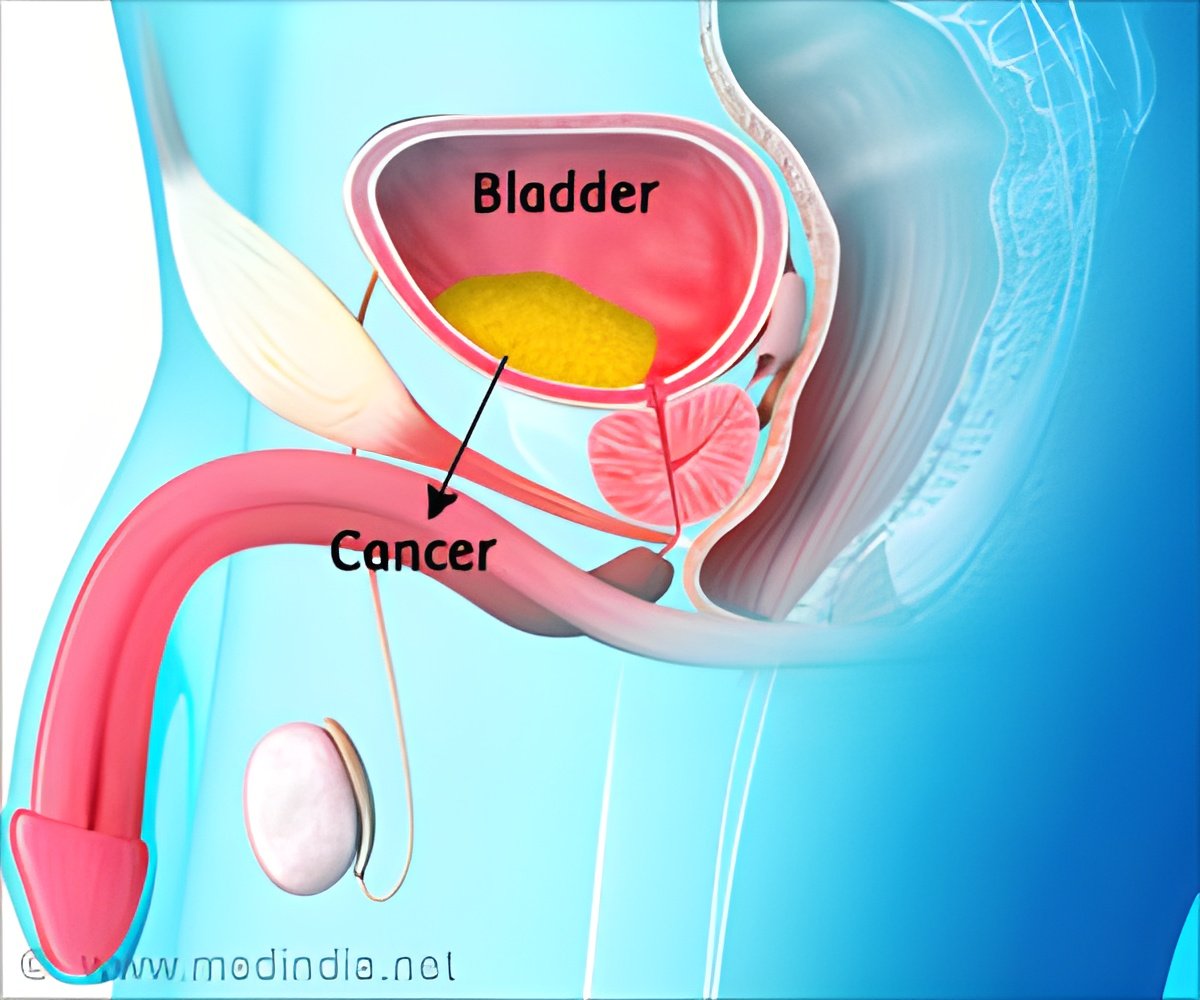
Living as a Bladder Cancer Survivor after Immunotherapy
These results, showing patients’ continued survival three years out, reinforce adjuvant nivolumab as a standard of care for patients with muscle-invasive urothelial cancer of the bladder or upper urinary tract. Normally, patients with this cancer face a high chance of recurrence, especially within the first three years after surgical removal of the bladder or kidney.‘Immunotherapy after surgery provides significant, durable benefits for high-risk bladder cancer patients.’





This new data showed that at approximately three years of follow-up, nivolumab increased these patients’ chances of staying cancer-free after surgery compared to patients who received a placebo.The average length of time before relapse doubled in patients who received nivolumab, which is a monoclonal antibody immune checkpoint inhibitor that harnesses the immune system to fight cancer. For a subset of clinical trial patients who received the immunotherapy, disease-free survival was more than six times that of patients on a placebo.
Among the 699 patients in the trial, half received nivolumab, and the other half received a placebo every two weeks for one year. Adjuvant nivolumab versus placebo was not associated with a detriment to the quality of life.
This trial was conducted with support from Bristol Myers Squibb, the maker of the immunotherapy, in collaboration with ONO Pharmaceutical Company Ltd.
Advertisement