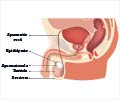
Link between advanced paternal age and higher risks for reproductive and offspring medical problems has been discovered.
‘Paternal age influences on the sperm methylome was found to affect offspring behavior and neurodevelopment.’





“we identified 1,162 (74%) regions which were significantly (FDR-adjusted) hypomethylated and 403 regions (26%) being hypermethylated with age.” Uncovering the Link Between Age-Related Methylation Changes and Human Sperm Epigenome There were no significant correlations with paternal BMI, semen quality, or ART outcome. The majority (1,152 of 1,565; 74%) of age-related differentially methylated regions (ageDMRs) were located within genic regions, including 1,002 genes with symbols. Hypomethylated ageDMRs were closer to transcription start sites than hypermethylated DMRs, half of which reside in gene-distal regions.
In this and conceptually related genome-wide studies, so far 2,355 genes have been reported with significant sperm ageDMRs, however most (90%) of them in only one study. The 241 genes which have been replicated at least once showed significant functional enrichments in 41 biological processes associated with development and the nervous system and in 10 cellular components associated with synapses and neurons.
The researchers found it interesting to note that sperm ageDMRs were not randomly distributed throughout the human genome; chromosome 19 showed a highly significant twofold enrichment with sperm ageDMRs.
Although the high gene density and CpG content have been conserved, the orthologous marmoset chromosome 22 did not appear to exhibit an increased regulatory potential by age-related DNA methylation changes.
Source-Eurekalert