Learn how low-intensity blood stem cell transplants improve lung function and offer new hope for adults with sickle cell disease.
‘Low-intensity #blood #stem_cell_transplants improve #lung_health in adults with #sickle_cell disease. #medindia’





Low-intensity blood stem cell transplants also known as non-myeloablative transplants, which use milder conditioning agents than standard stem cell transplants, do not appear to harm the lungs and may help improve lung function in some sickle cell disease (SCD) patients, according to a three-year study of adults who underwent the procedure at the National Institutes of Health.Low-Intensity Stem Cell Transplants
"By using a low-intensity blood stem cell transplant for sickle cell disease, we may be able to stop the cycle of lung injury and prevent continued damage," said study lead Parker Ruhl, M.D., an associate research physician and pulmonologist at NIH. "Without the ongoing injury, it’s possible that healing of lung tissue might occur, and this finding should help reassure adults living with sickle cell disease who are considering whether to have a low-intensity stem cell transplant procedure that their lung health will not be compromised by the transplant (1✔ ✔Trusted SourcePulmonary Function After Non-Myeloablative Hematopoietic Cell Transplant for Sickle Cell Disease
Go to source)."
Until recently, bone marrow and blood stem cell transplants were the only cure for sickle cell disease, but relatively few adults have undergone the treatments due to health risks associated with high doses of chemotherapy required to prepare for transplants. In addition, the process requires a genetically well-matched donor, usually a sibling who does not have SCD.
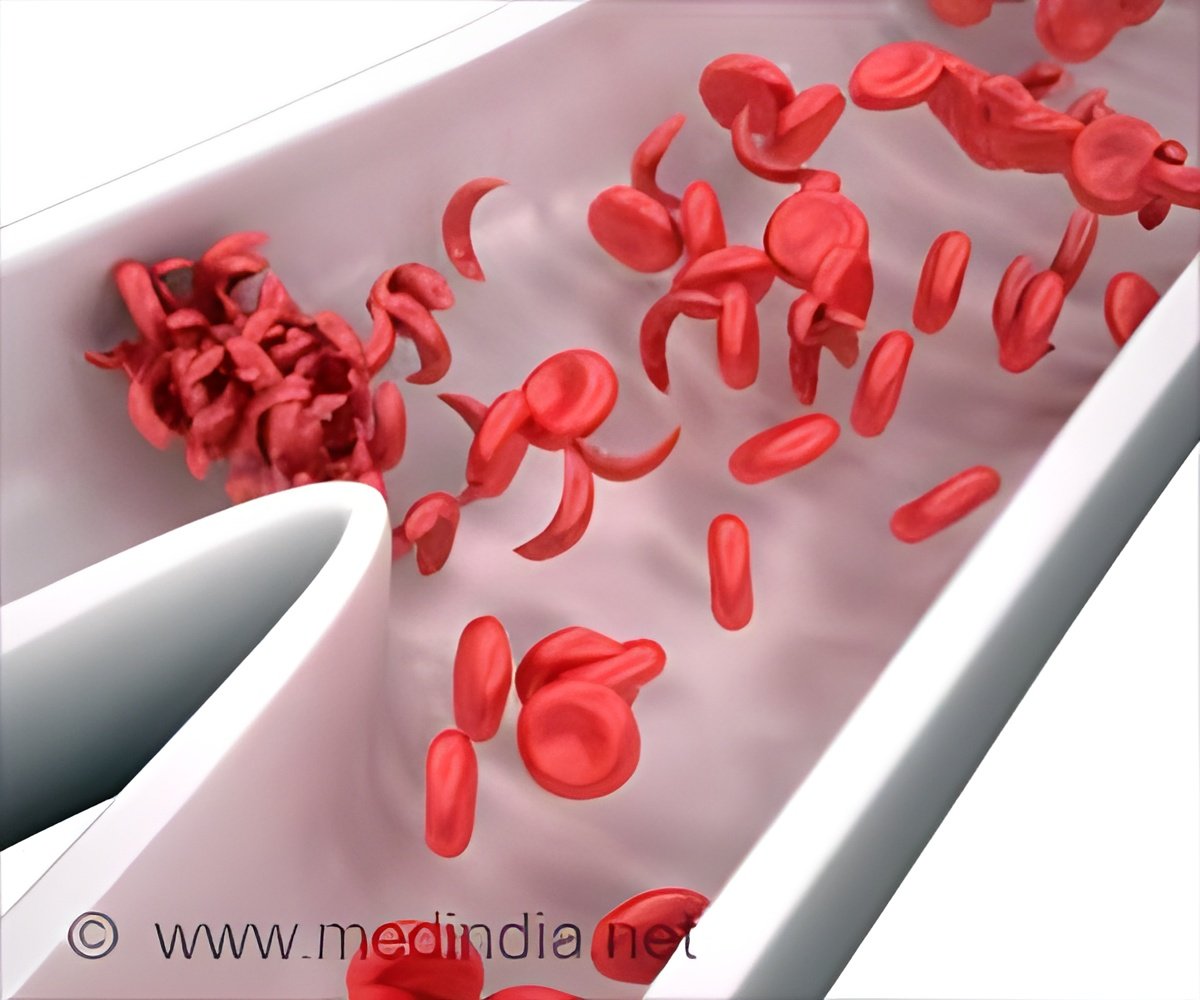
These procedures involve giving patients blood stem cells obtained from a donor to grow normal red blood cells to replace the "sickled" cells. The sickled cells block blood flow throughout the body, causing a host of problems, including episodes of acute pain, infections, stroke, and acute chest syndrome, in which the lungs are deprived of oxygen.
Researchers say at least one-third of the sickle cell stem cell transplants performed are low-intensity. While low-intensity blood stem cell transplant is slightly less effective than the standard transplants, adults who often have more pre-existing organ damage than children tend to do better with them and also experience a lower risk for complications such as graft-versus-host disease. The current study examined if these transplants offered other benefits for adults with already vulnerable lungs.
Advertisement
Assessing Lung Health with Pulmonary Function Tests
For the research, Ruhl and her team studied 97 patients with sickle cell disease who underwent a low-intensity, or non-myeloablative, blood stem cell transplant between 2004 and 2019 at the NIH’s Clinical Center in Bethesda, Maryland. Participants were then followed for up to three years.The researchers conducted various pulmonary function tests, including
- Forced expiratory volume in one second (FEV-1) which measures the amount of air exhaled in the first second after forced exhalation.
- Lung diffusion test, or diffusing capacity of the lungs for carbon monoxide (DLCO), which measures how much oxygen moves from the lungs to the blood when exhaling.
Ruhl said that larger studies with longer follow-up periods and the inclusion of transplant data from other clinical centers, including those from patients who received a standard transplant, are still needed to put the current findings in context. In the meantime, she and her team will continue to follow the NIH patients and report on longer-term outcomes at the five- and 10-year mark.
In December 2023, the U.S. Food and Drug Administration (FDA) approved two genetic therapies that use patients’ blood stem cells to treat SCD. Researchers hope that the techniques used in this study will also be used to evaluate lung function for other new genetic therapies.
Reference:
- Pulmonary Function After Non-Myeloablative Hematopoietic Cell Transplant for Sickle Cell Disease - (https:www.atsjournals.org/doi/10.1513/AnnalsATS.202309-771OC)
Source-Eurekalert