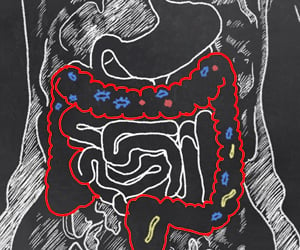
The way in which the microbes that are closest to our hearts could underpin a new way of thinking about human biology will be explained by world class scientist Professor Willem M. de Vos.
Gut microbes affect our health by producing vitamins, priming our immune system and contributing to resistance to pathogens. For example, recent studies have shown that the insulin resistance of patients with type 2 diabetes is linked to the intestinal microbiota composition and can be beneficially altered by replacing it with the microbiota of healthy donors.
The genes of our gut microbes, also known as the microbiome, act as a personalized organ that can be modified by diet, lifestyle and antibiotics. This organ is fed partly by us and partly by our diets. Professor de Vos and colleagues have classified the human microbiome into three enterotypes: clusters of microbiomes with similar compositions and nutrient-processing preferences. These enterotypes are characterized by bacteria with different capacities to degrade carbohydrate and mucin (a gel-forming protein which produces mucus). Our gut microbes get carbohydrates partly from our diet, whereas the mucin is produced by our own body.
Although these enterotypes are separated by species composition, it doesn't necessarily follow that abundant functions are provided by abundant species. To investigate the relationship between the microbiome and health, scientists must establish the functions of the products of their microbiomes.
"We have evolved with the microbes in our gut, our microbes inside, and have discovered that they talk to us and we feed them with, among other things, the mucins we produce. We now are trying to unravel their functions and understand exactly what these microbes and their products mean to human health" said Professor de Vos.
The size of one microbial metagenome (one host's microbiome) is 150 times larger than the human genome and encodes 100 times more genes than our own genome. This extensive gene catalogue could enable us to study potential associations between microbial genes and human phenotypes and even environmental factors like diet, throughout the length of our lifetime.
Advertisement
Advertisement