Researchers reports that a single injection of human neural stem cells produced neuronal regeneration and improvement of function and mobility in rats impaired by an acute spinal cord injury (SCI).
The findings are published in the May 28, 2013 online issue of Stem Cell Research & Therapy.
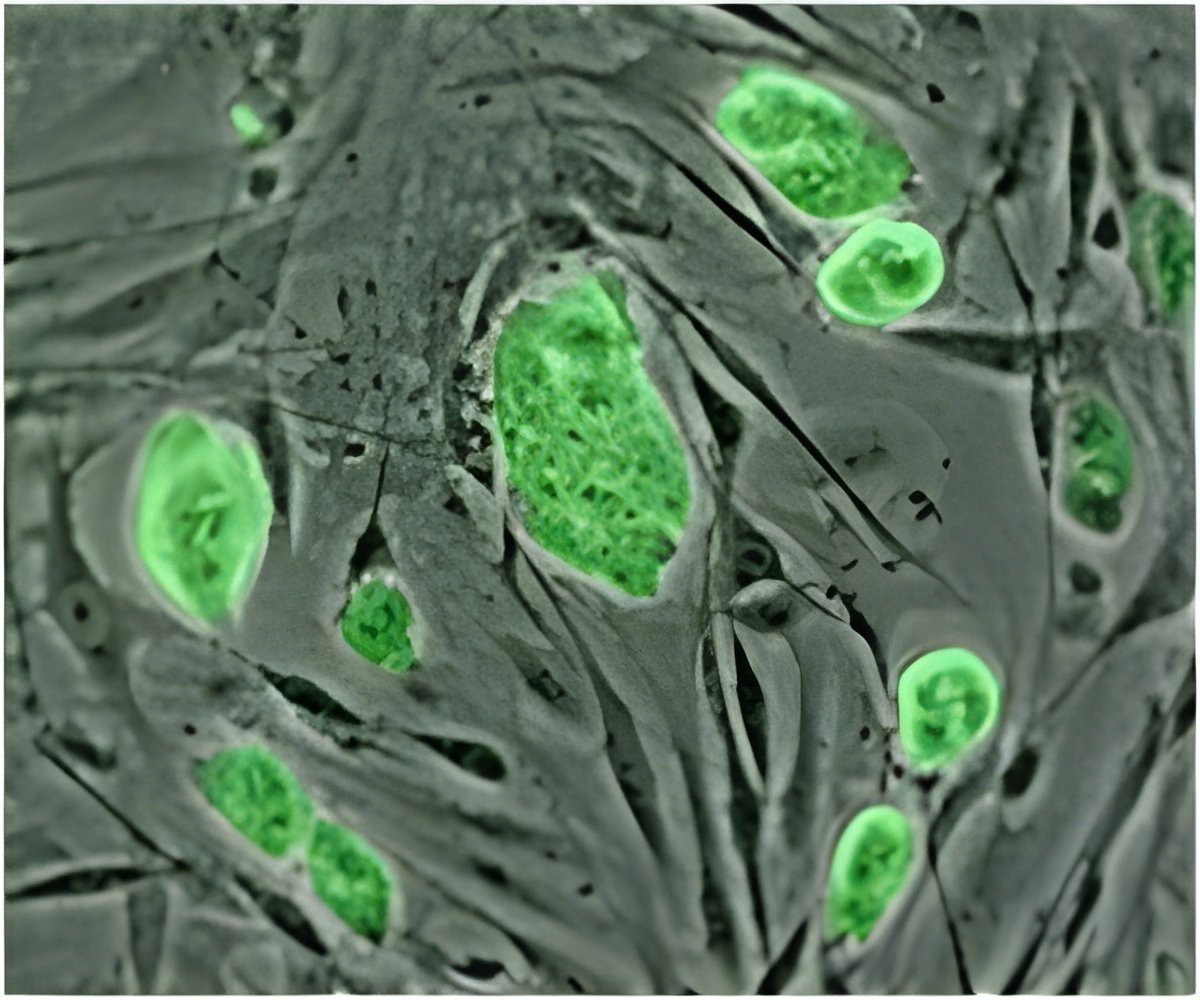
Martin Marsala, MD, professor in the Department of Anesthesiology, with colleagues at UC San Diego and in Slovakia, the Czech Republic and The Netherlands, said grafting neural stem cells derived from a human fetal spinal cord to the rats spinal injury site produced an array of therapeutic benefits - from less muscle spasticity to new connections between the injected stem cells and surviving host neurons.
"The primary benefits were improvement in the positioning and control of paws during walking tests and suppression of muscle spasticity," said Marsala, a specialist in spinal cord trauma and spinal injury-related disorders. Spasticity - exaggerated muscle tone or uncontrolled spasms - is a serious and common complication of traumatic injury to the spinal cord.
The human stem cells, said the scientists, appeared to vigorously take root at the injury site.
"In all cell-grafted animals, there was robust engraftment, and neuronal maturation of grafted human neurons was noted," Marsala said. "Importantly, cysts or cavities that can form in or around spinal injuries were not present in any cell-treated animal. The injury-caused cavity was completely filled by grafted cells."
Advertisement
The grafted stem cells, according to Marsala, appear to be doing two things: stimulating host neuron regeneration and partially replacing the function of lost neurons.
Advertisement
The scientists used a line of human embryonic stem cells recently approved for Phase 1 human trials in patients with chronic traumatic spinal injuries. Marsala said the ultimate goal is to develop neural precursor cells (capable of becoming any of the three main cell types in the nervous system) from induced pluripotent stem cells derived from patients, which would likely eliminate the need for immunosuppression treatment.
Pending approval by UC San Diego's Institutional Review Board, the next step is a small phase 1 trial to test safety and efficacy with patients who have suffered a thoracic spinal cord injury (between vertebrae T2-T12) one to two years earlier, and who have no motor or sensory function at or below the spinal injury site.
"This is exciting, especially because, historically, there has been very little to offer patients with acute spinal cord injury," said study co-author Joseph Ciacci, MD, professor of surgery and program director of the Neurosurgery Residency at the UC San Diego School of Medicine. Ciacci, who is also chief of neurosurgery for the Veterans Affairs San Diego Healthcare System, will oversee the clinical trial at UC San Diego and the VA.
Ciacci said if the initial study confirms safety and efficacy, as well as the viability of the implanted cells, neural regeneration and decreased spasticity, the protocol can be expanded to other patients with other forms of severe spinal cord injury.
For further information about the planned phase 1 trial, contact Adrienne Rebollo at [email protected].
Co-authors are Sebastiaan van Gorp, UCSD Department of Anesthesiology and Department of Anesthesiology, School for Mental Health and Neurosciences, Maastricht University Medical Center, The Netherlands; Marjolein Leerink, Osamu Kakinohana, Olexandr Platoshyn, Camila Santucci, Danielle Goldberg and Silvia Marsala, UCSD Department of Anesthesiology; Jan Galik, Institute of Neurobiology, Slovak Academy of Sciences and Institute of Biology and Ecology, Pavol Jozef Safarik University, Slovakia; Elbert A. Joosten, Department of Anesthesiology, School for Mental Health and Neurosciences, Maastricht University Medical Center, The Netherlands; Marian Hruska-Plochan, UCSD Department of Anesthesiology, Institute of Animal Physiology and Genetics, Czech Academy of Sciences and Department of Cell Biology, Charles University, Czech Republic; Karl Johe, Neuralstem, Inc., Rockville, Md.
Funding came, in part, from the California Institute for Regenerative Medicine (RM1-01720) and Neuralstem Inc., which also provided cells.
Source-Newswise