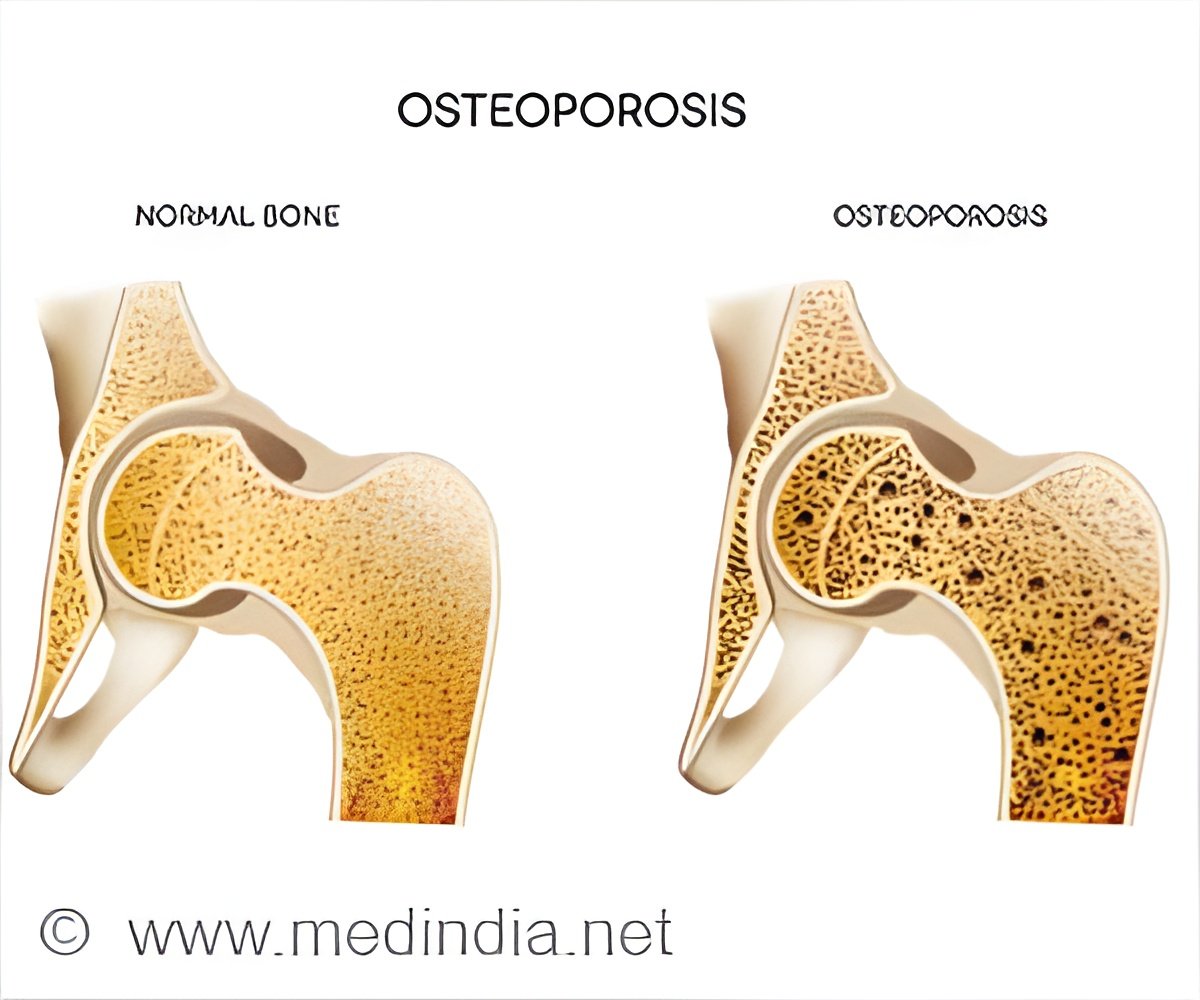
A recent study provides evidence endorsing the Osteoboost's efficacy in alleviating the reduction in bone strength and density among postmenopausal women.
BONE HEALTH TECHNOLOGIES TO PRESENT CLINICAL DATA AT ASBMR 2023 FROM PIVOTAL TRIAL OF WEARABLE TREATMENT FOR OSTEOPENIA IN POST-MENOPAUSAL WOMEN
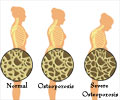
Go to source) Public health data shows that most fragility fractures occur in patients with osteopenia, further emphasizing the urgent need for effective early interventions before patients progress to osteoporosis.
‘In postmenopausal women, Osteoboost a novel wearable device led to an 83% reduction in vertebral bone strength loss within one year among participants following the prescribed protocol. #bonehealth #worldosteoporosisday #osteopenia ’





Bone Health Technologies has filed for Class 2 Prescription De Novo Approval with the FDA and was previously awarded Breakthrough Device Status.
Osteoboost Study: Efficacy in Mitigating Bone Strength and Density Decline in Postmenopausal Women
BHT conducted a study at the University of Nebraska Medical Center involving 126 postmenopausal women with low bone mass who were not taking bone-active medications. The research followed a double-blinded, sham controlled design. The findings presented provided compelling evidence supporting the effectiveness of the Osteoboost in mitigating the decline of bone strength and bone density in this demographic.The primary goal of the study was to provide quantifiable measurement in the change in vertebral strength using Biomechanical Computed Tomography and finite element analysis. The participants in the Active Treatment group who used the device a minimum of 3 times per week throughout the year experienced an average bone strength loss of 0.48%. In contrast, those in the Sham group lost 2.84% on average, indicating a relative difference of 2.36% (P=0.014). This represents an impressive 82% reduction in the rate of bone strength loss among the Active Treatment group.
“Patients with osteopenia lack clinically-proven interventions to slow or reverse their loss of bone – we are excited by the trial results showing effectivess of the first non-pharmacological intervention to slow loss of bone strength as measured by CT,” said Laura Yecies, CEO of Bone Health Technologies.
When transitioning through menopause, women face accelerated bone loss due to the rapid loss of estrogen. While there are pharmaceutical interventions, they are not typically prescribed to those with osteopenia. The Osteoboost is unique as an easy to use, clinically proven and safe intervention for osteopenia. The belt is engineered to enhance bone strength and density by emitting targeted vibrations to the lumbar spine and hips, and providing a safe and efficient treatment option.
Following a thorough screening process, including DXA scans and a comprehensive blood workup, participants were divided into two randomized groups: the Active group received Osteoboost treatment five times per week for 12 months, while the Sham group used the same device but received a placebo treatment. Compressive strength and volumetric density of the first lumbar vertebrae (L1) were measured using Finite Element Analysis of computed tomography (CT) scans of the lumbar spine by ON Diagnostics.
Advertisement
Reference:
- BONE HEALTH TECHNOLOGIES TO PRESENT CLINICAL DATA AT ASBMR 2023 FROM PIVOTAL TRIAL OF WEARABLE TREATMENT FOR OSTEOPENIA IN POST-MENOPAUSAL WOMEN - (https://www.bonehealthtech.com/news/press-release/988/)