A new drug derived from magnolia trees appears to be able to uncouple two important functions of thrombin in blood clot formation and may offer a way to better control.
A new drug derived from magnolia trees appears to be able to uncouple two important functions of thrombin in blood clot formation and may offer a way to better control the potentially dangerous complications of bleeding and clot formation during procedures to open blocked coronary arteries, say researchers at the Duke Clinical Research Institute (DCRI).
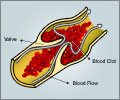
Results of the Phase II study of the drug, known as SCH 530348, appear online in the journal The Lancet and will appear in the publication's Mar. 14 issue. An international, Phase III study is already under way.Thrombin is a protein in the blood that performs two key functions in clot formation: It activates platelets, particles that clump together to form the scaffolding of a clot, and it helps produce fibrin, a protein necessary in repairing damaged tissue.
"One of the intriguing things about this new investigational compound is that it blocks the thrombin receptor that activates platelet formation yet preserves the beneficial activity of fibrin formation," said Richard Becker, M.D., a cardiologist at DCRI and the lead author of the study. "And the data to date indicated the compound does this even when patients are taking aspirin and clopidogrel."
Blood clots are a big issue for patients facing percutaneous coronary intervention (PCI) the process of clearing clogged arteries with a balloon and then propping them open with stents. Patients who undergo PCI are usually prescribed anti-clotting agents like aspirin or clopidgrel (Plavix). Becker said while those drugs are effective, they can sometimes lead to bleeding, and with current therapy, the rate of additional cardiovascular events remains quite high. "So there is a very aggressive search going on for better and safer agents," he said.
In the current study, researchers randomly assigned 1030 patients 45 years or older scheduled to undergo non-urgent PCI or angiography with planned PCI to a group that would receive one of three escalating doses of SCH 530348 (10 mg, 20 mg or 40 mg), or to a control group, which took a placebo. Both researchers and patients were both blinded to who was taking which drug.
Aspirin and clopidogrel were not prescribed as part of the study – investigators followed standard practice at each study site and found that 95 percent of the patients were given both aspirin and clopidogrel.
Advertisement
They found that SCH 530348 was generally tolerable at all dosing levels and did not increase bleeding, even when taken along with aspirin and clopidogrel. Bleeding occurred in 2 percent of 129, 3 percent of 120, and 4 percent of 173 patients in the 10 mg, 20 mg, and 40 mg drug groups, respectively; compared with 3 percent of the 151 patients in the placebo group (p = .5786).
Advertisement
"The uncoupling of benefit and bleeding risk among patients with acute coronary syndromes is an important objective in drug development," Becker said. "We will need to see if Phase III clinical trials can confirm these findings."
Source-Eurekalert
SRM