Researchers isolated egg-producing stem cells from human ovaries and made them generate egg cells.
"The prevailing dogma in our field for the better part of the last 50 or 60 years was that young girls at birth were given a bank account of eggs at birth that's not renewable," said Jonathan Tilly, director of the Vincent Center for Reproductive Biology at Massachusetts General Hospital, who led the research.
"As they become mature and become a woman, they use those eggs up (and) the ovaries will fail when they enter menopause."
Tilly first challenged the "bank account" doctrine eight years ago, suggesting female mammals continue producing egg-making cells into adulthood rather than from a stock acquired at birth.
His theory ran into a firestorm.
Other scientists challenged the accuracy of his experiments or dismissed their conclusions as worthless, given that they had only been conducted on lab mice.
Advertisement
In it, his team isolated egg-producing stem cells in human ovaries and then coaxed them into developing oocytes, as eggs are called.
Advertisement
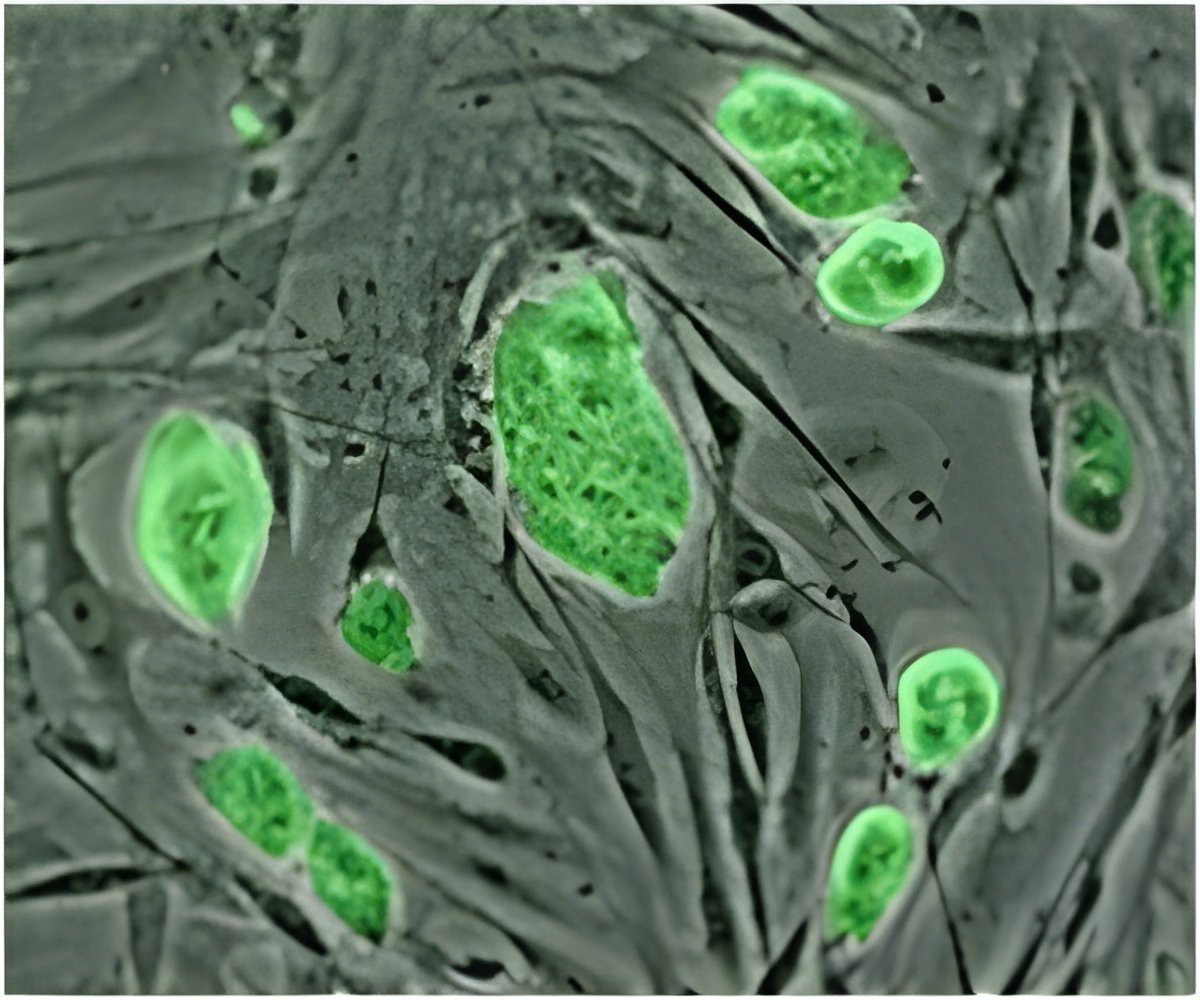
The team tagged the stem cells with a fluorescent green protein -- a common trick to help figure out what happens in lab experiments.
The cells were injected into biopsied human ovarian tissue which was then grafted beneath the skin of mice.
Within 14 days, the graft had produced a budding of oocytes. Some of the eggs glowed with the fluorescent tag, proving that they came from the stem cells. But others did not, which suggested they were already present in the tissue before the injection.
Tilly said "the hairs were standing up on my arm" when he saw time-elapse video showing the eggs maturing in a lab dish.
Further work needs to be done to test the viability of the eggs, and little is known about the hormones or other mechanisms by which oocytes emerge from the stem cells.
But the impact could be far-reaching, Tilly said.
"If we can guide the process correctly, I think it opens up a chance that sometime in the future, we might get to the point of actually having an unlimited source of human eggs," Tilly said in a video recording released to the press.
"A woman could come in, have a small biopsy taken from her ovary for us to retrieve these cells. Once we get these cells out, we can take a hundred of them and make a million of them.
"If we can get to the stage of generating functional human eggs outside the body, it would rewrite essentially human assisted reproduction."
According to a press release issued by Massachusetts General Hospital, Tilly's team are already exploring the idea of banks where oocyte stem cells can be frozen and stored, and then retrieved when a woman wants to have a baby.
Human eggs are extremely delicate and likely to suffer damage when frozen and thawed, but this risk does not apply to the egg cells that make them, it said.
Previous work has shown that around one in 10 women of reproductive age is at risk of premature ageing of the ovaries, a finding with repercussions in societies where women opt ever later to become mothers.
Source-AFP