A new method based on the principle of enzyme inhibition measures how quickly drugs interact with their molecular targets.
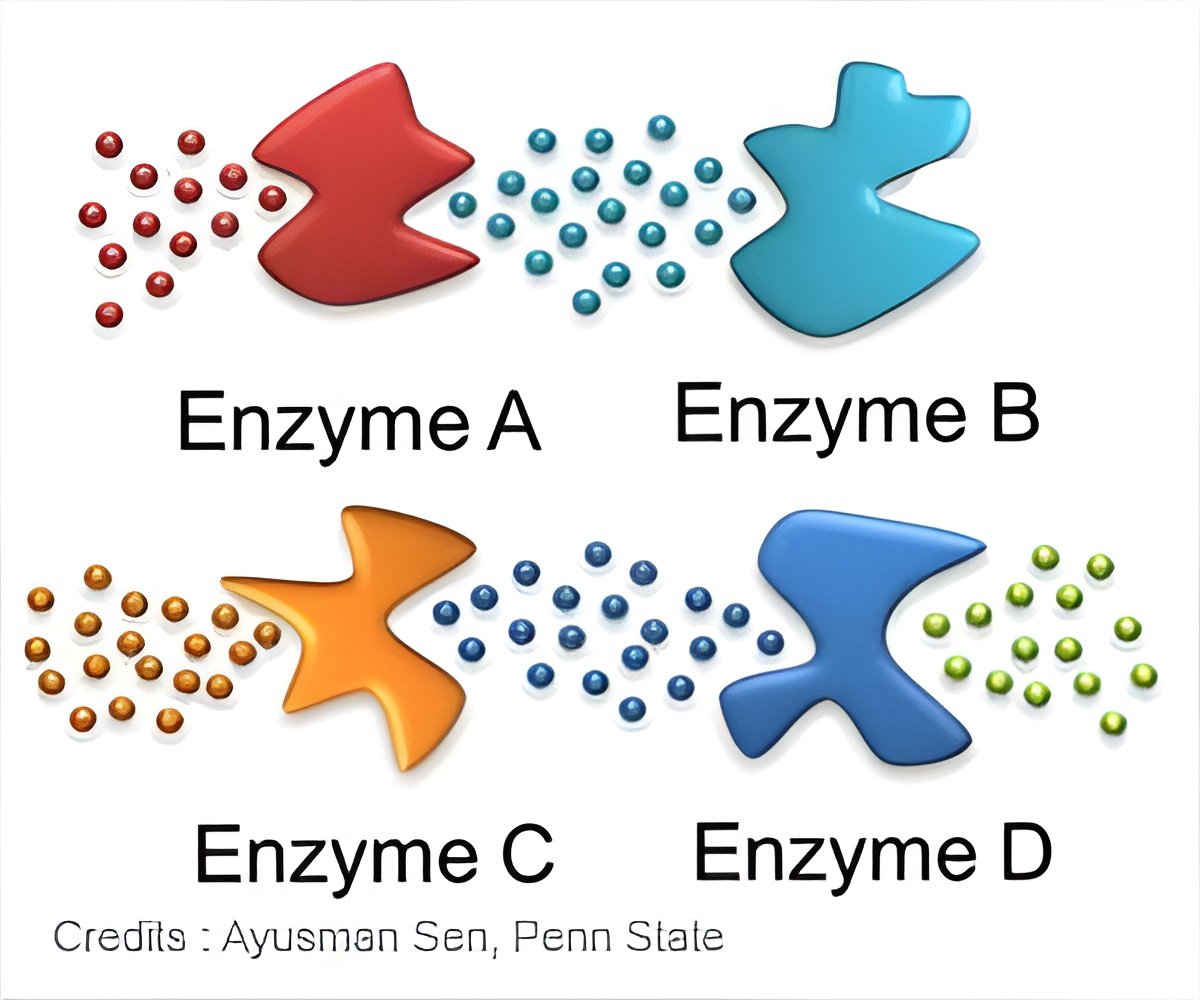
‘Isothermal titration calorimetry (ITC) technique measures how quickly drugs interact with molecular targets and offers a new approach to drug discovery.’





In a paper published in Nature Communications, the McGill team, led by chemistry professors Nicolas Moitessier and Anthony Mittermaier, demonstrate the use of isothermal titration calorimetry (ITC) to measure the heat generated by enzyme activity and thereby the rates at which inhibitor substances blocked that activity. "One key difference between ITC and other methods is that ITC measures the rate of reaction directly," Mittermaier explains.
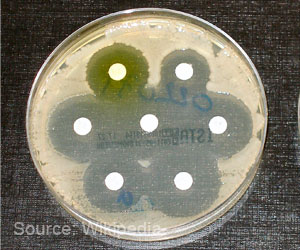
Existing methods for measuring enzyme activity look at that activity indirectly, by measuring changes in concentration caused by enzymatic catalysis as a function of time. These measurements often depend on special reagents that change colour or fluorescence when acted on by the enzyme, and require a unique test to be developed for each enzyme being studied.
Because ITC measures the production of heat - a near-universal feature of chemical reactions - it can be applied to just about any enzyme.
"ITC is as close as you can get to a universal enzyme test," Mittermaier says.
Advertisement
The real-time nature of ITC is particularly promising for researchers investigating covalent inhibitors. These strongly binding molecules have potential as long-acting drugs but had previously fallen out of favour in drug development due to toxicity concerns. The insight ITC offers into the relationship between an inhibitor's molecular structure and how it reacts with its target will support renewed interest in covalent inhibitors and facilitate the work of developing them into drugs that are both highly effective and safe.
Advertisement
Source-Eurekalert