New oral interleukin 23 receptor (IL-23R) antagonist drug for treating moderate-to-severe plaque psoriasis is set to reach global sales of $292 million by 2029.
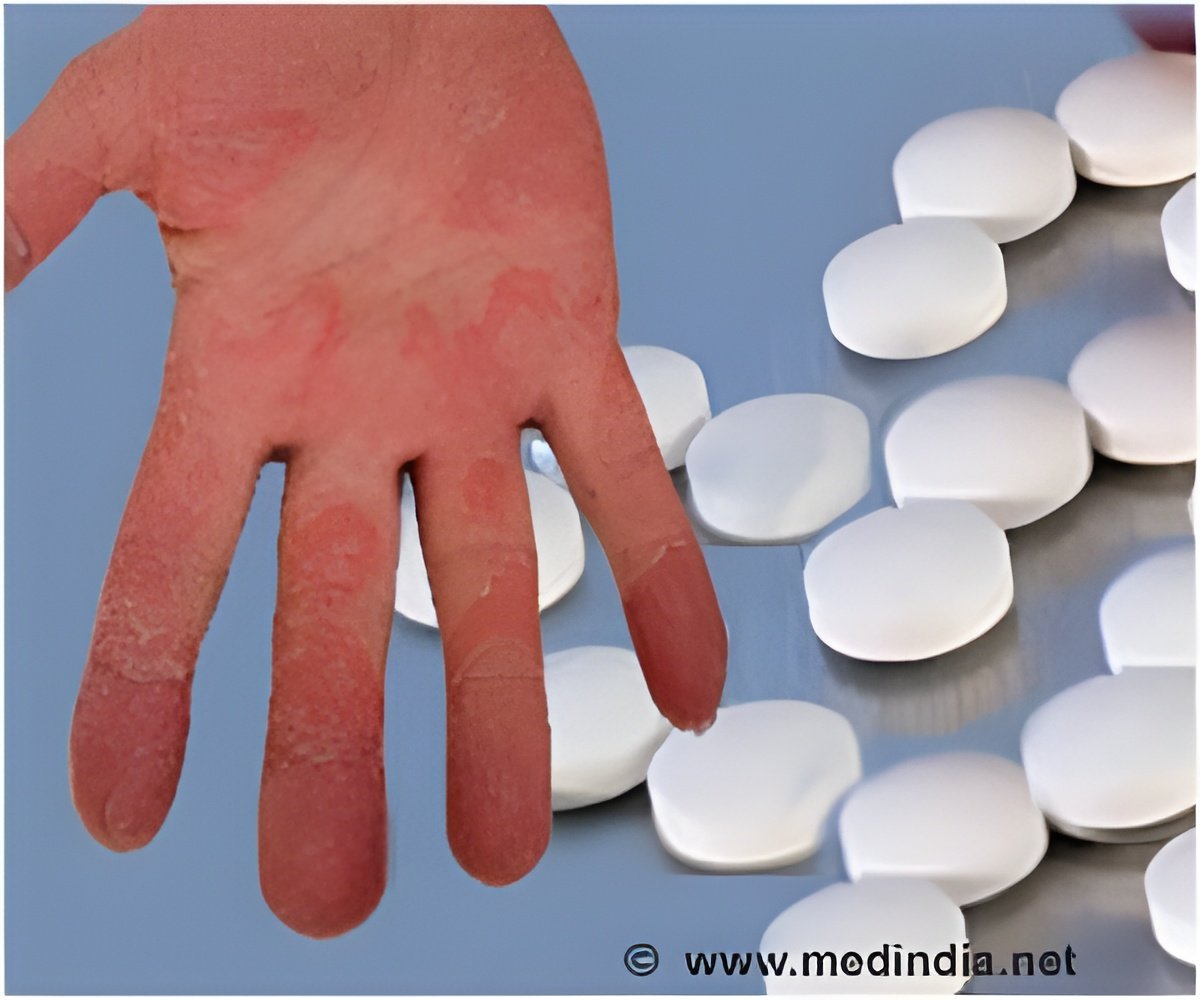
‘JNJ-2113 is an innovative oral therapy that specifically targets interleukin 23 receptor (IL-23R) and has the potential to become the leading treatment for psoriasis.#Psoriasis #ProtagonistJNJ 2113 #JanssenPharmaceuticals’





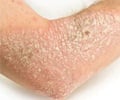
Psoriasis is a long-term skin disease that causes a rash with itchy, scaly patches in the knees, elbows, trunk, and scalp. It can be painful, interfere with sleep, and make it hard to concentrate.The treatment aims to stop skin cells growth quickly for removing scales. The options include creams, ointments, light therapy (phototherapy), and oral or injected medications (1✔ ✔Trusted Source
Psoriasis: a brief overview
Go to source). The treatment used depends on the severity of psoriasis, the response to previous treatment, and self-care measures.
Janssen Develops New Oral Drug JNJ-2113 for Moderate-to-Severe Psoriasis
The potential of JNJ-2113 was demonstrated in phase two clinical trial data. It revealed complete clearance and improvement in skin lesions in the participants when compared to the respective placebo groups.The efficacy of JNJ-2113 was reinforced again by the results of Johnson & Johnson’s (J&J) randomized, multicenter, double-blind, placebo-controlled, 24-week long Phase two FRONTIER 1 (NCT05223868) clinical trial.
This clinical trial analyzed three once-daily dosages and two twice-daily dosages taken orally by patients with moderate-to-severe psoriasis. This laid the basis for the agent’s progression to Phase III development (2✔ ✔Trusted Source
Comparative safety and benefit-risk profile of biologics and oral treatment for moderate-to-severe plaque psoriasis: A network meta-analysis of clinical trial data
Go to source).
The results showed that the Psoriasis Area and Severity Index (PASI) 75 was 9.3% for the placebo group, and 37.2%, 58.1%, 51.2%, 65.1%, and 78.6% for the JNJ-2113 groups at week 16, respectively. Similarly, PASI 90 was 2.3% for the placebo group, and 25.6%, 51.2%, 26.8%, 46.5%, and 59.5% for the treatment groups at week 16, respectively. This demonstrates dose-dependent responses across primary and secondary endpoints.
Advertisement
The promising results of the flexible and convenient agent signify the potential impact of JNJ-2113 on the treatment paradigm for moderate-to-severe psoriasis. However, indirect comparisons should be made with caution due to differences in clinical trial designs and patient populations.
With advanced psoriasis treatments having limited to injectable biologics, the data underlines the ability and potential of JNJ-2113 to offer an opportunity to address patient needs and preferences by providing an oral therapy that directly targets the IL-23 pathway. This may improve patient outcomes.
References:
- Psoriasis: a brief overview - (https://www.rcpjournals.org/content/clinmedicine/21/3/170)
- Comparative safety and benefit-risk profile of biologics and oral treatment for moderate-to-severe plaque psoriasis: A network meta-analysis of clinical trial data - (https://pubmed.ncbi.nlm.nih.gov/33631216/)
Source-Medindia