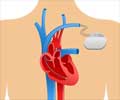
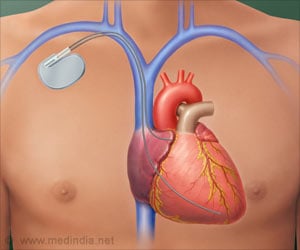
Does the pacemaker affect drugs acting on the heart? Modulating the interaction between the pacemaker and fat molecules with a drug could improve heart disease treatment.
‘Understanding precisely how pacemaker channels work could lead to better treatments for cardiac arrhythmias, chronic pain, epilepsy, and other conditions.’





Ion channels have been notoriously difficult to target with drugs, because of the challenge of identifying drug-binding sites that are specific to particular channels. But this work reveals sites that might be highly specific and thus viable drug targets.How To Improve Heart Disease Treatment With Pacemaker?,
Ion channels are tubelike protein structures that reside within the membranes of cells, enabling and regulating flows of charged molecules of potassium, sodium, and other electrolytes into and out of the cell. These flows of charged molecules, or ions, are key determinants of cell behavior.Specialized ion channels called pacemaker channels are particularly important for the rhythmic activities of heart cells and neurons. Understanding precisely how these pacemaker channels work could thus lead to better treatments for cardiac arrhythmias, chronic pain, epilepsy, and other conditions. Arrhythmias alone is estimated to affect millions of people in the United States.
Scientists have known that lipids, the major constituents of cell membranes, are involved in modulating the activities of pacemaker channels. But they haven’t known much about how these interactions work.
Researchers were able to obtain important clues about lipid-pacemaker channel interactions by first examining a bacterial pacemaker channel, SthK. In prior studies, they had developed an experimental platform for studying SthK, including a membrane-like environment that they could alter experimentally.
SthK is a useful model for pacemaker channels found in humans—known as HCN channels—because the two channels, despite the evolutionary gulf between them, have many important similarities.
Advertisement
When the salt bridge was disrupted by the lipids, the channel became more open and active. Similarly, removing the salt bridge by other means dramatically increased channel activity and abolished the ability of these lipids to affect that activity.
Advertisement
The experiments included cryo-EM imaging of lipids binding to SthK—imaging that offers clues to how a future drug might disrupt or enhance this lipid interaction to modulate pacemaker channel function.
Researchers hope that in follow-on work they can illuminate the role of this lipid-pacemaker interaction in abnormal conditions such as arrhythmias or cancers.
Various conditions can affect the lipid composition of the heart and other tissues, so it wouldn't be surprising to see this pacemaker mechanism altered in diseases.
Source-Eurekalert