L-glutamine has been approved by the US FDA for the treatment of sickle cell disease to prevent complications.
- Patients with sickle cell disease have limited treatment options
- L-glutamine protects red blood cells in patients with sickle cell disease and reduces hospitalizations
- It can be used in children as young as 5 years of age
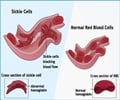
Patients with sickle cell disease have abnormal hemoglobin. If the oxygen levels are low, the disc-shaped red blood cells assume a sickle shaped. The shape does not allow them to pass through blood vessels easily and they can therefore obstruct the flow of blood to various organs. Patients suffer from frequent severe painful episodes, which often require painkiller injections. The cells also have a tendency to break, as a result of which anemia ensues. Currently, hematopoietic stem cell transplant is the definitive treatment for sickle cell disease, though it may be beyond the reach of several patients.
A medication called hydroxyurea is used for the treatment of sickle cell disease. It increases the levels of a type of hemoglobin called fetal hemoglobin, and therefore reduces the complications of sickle cell disease.
Studies found that the red blood cells affected by sickle cell disease are under high oxidative stress and try to protect themselves by producing a chemical called nicotinamide adenine dinucleotide (NAD) molecules. The newly approved drug, l-glutamine, is an amino acid required to produce NAD molecules, and can therefore protect the red blood cells.
L-glutamine can be taken as an oral powder by sickle cell disease patients as young as 5 years of age. The approval comes following the results of a trial, which compared the use of L-glutamine to a placebo taken for a duration of 48 weeks. The participants of the trial had previously suffered at least two attacks of painful crises in the previous year. The patients who took the medication had:
- Fewer visits to the hospital for painkiller injections for painful episodes
- Fewer hospitalizations
- Reduced duration of hospitalizations
- Fewer incidences of acute chest syndrome. Acute chest syndrome is a condition where obstruction to blood flow to the lungs can damage the lungs
The new drug will hopefully be a boon to several patients affected by sickle cell disease.
- FDA approves new treatment for sickle cell disease - (https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm566084.htm)
- What is Sickle Cell Disease - (https://www.nhlbi.nih.gov/health/health-topics/topics/sca)
- Niihara Y, Macan H, Eckman JR, Koh H, Cooper ML, et al. (2014) L-Glutamine Therapy Reduces Hospitalization for Sickle Cell Anemia and Sickle β°-Thalassemia Patients at Six Months – A Phase II Randomized Trial. Clin Pharmacol Biopharm 3:116. doi:10.4172/2167-065X.1000116