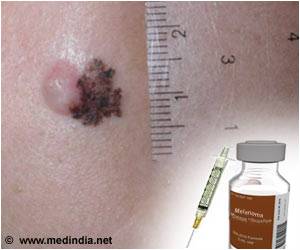
Lasting remissions can be produced by a drug that unleashes the immune system to attack cancer and hold the disease in check for more than two years in some cases, in patients with advanced melanoma.
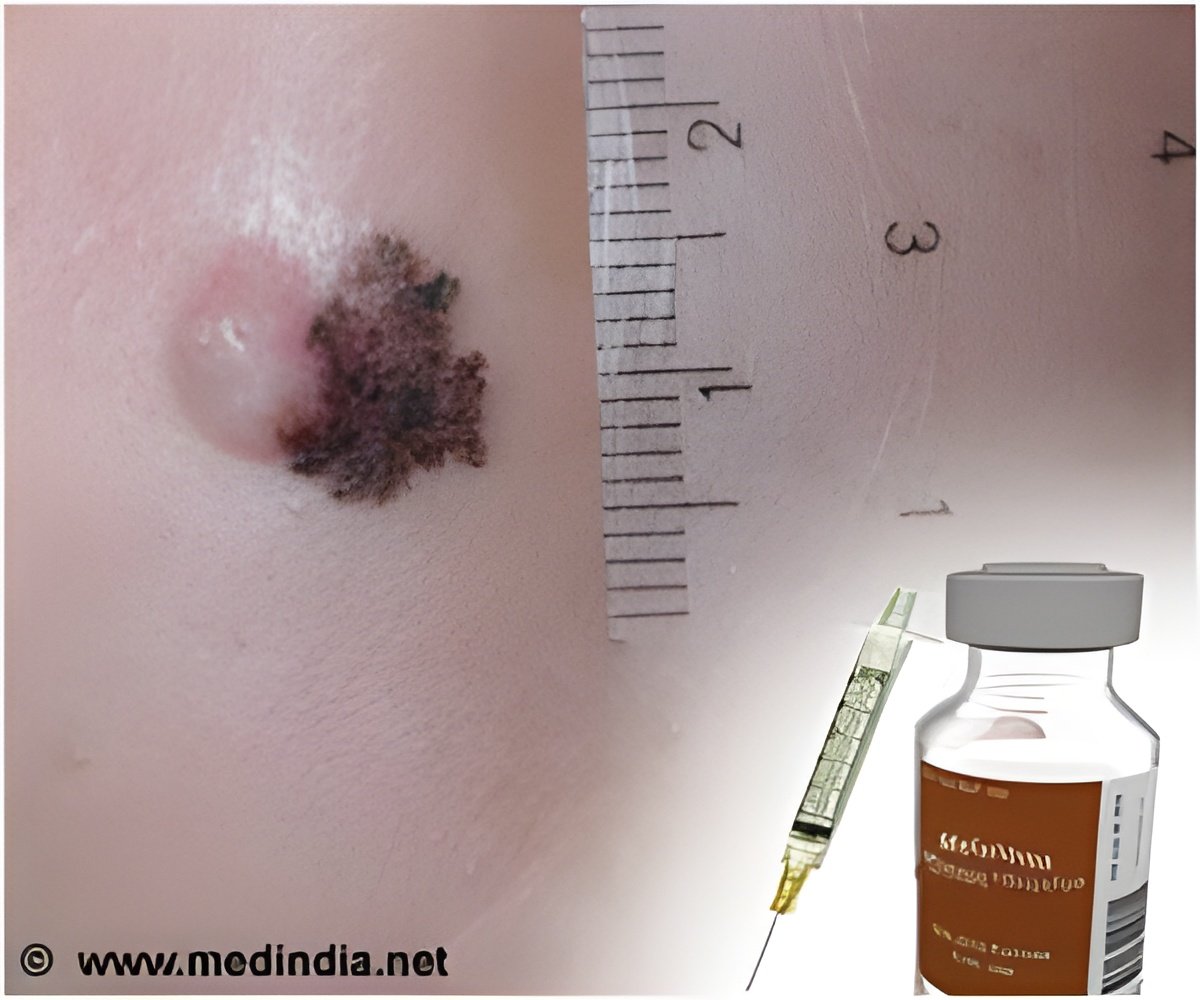
The clinical trial involved 107 patients with advanced melanoma that no longer responded to other treatments. They received nivolumab intravenously every other week for up to 96 weeks. After one year, 62 percent of the patients were alive. After two years, 43 percent were alive.
"These are striking results for patients with metastatic melanoma," said the study's senior author Stephen Hodi, MD, director of the Melanoma Treatment Center at Dana-Farber. "This study provides the first demonstration of the long-term benefits this treatment approach can produce."
Nivolumab is one of a new generation of drugs that essentially releases the brakes on an immune system attack on cancer. The drug blocks PD-1, a protein on immune system T cells that restrains them from leading a charge on tumor cells. By interfering with PD-1, nivolumab allows the attack to proceed.
The side effects of the treatment were consistent with those observed in previous studies of the drug. The most common adverse experiences were fatigue, rash, and diarrhea, most of which occurred in the first six months of therapy. Prolonged exposure to the drug didn't produce cumulative negative effects, the investigators report.
One of the most noteworthy findings of the study is that, in many patients, tumors either remained the same size or continued to recede even after the treatment period was over. "This suggests that blockade PD-1 may reset the equilibrium between the immune system and the tumor, keeping tumor growth in check," remarked Hodi, who is also the director of the Center for Immuno-Oncology at Dana-Farber.
Advertisement
The effectiveness of nivolumab in these patients suggests that combining the drug with other immune system-based therapies may prove even more potent, the authors indicate. Clinical trials are already under way to assess such a combined approach.
Advertisement
Funding for the study was provided by Bristol-Myers Squibb.
About Dana-Farber Cancer Institute
Dana-Farber Cancer Institute (www.dana-farber.org) is a principal teaching affiliate of the Harvard Medical School and is among the leading cancer research and care centers in the United States. It is a founding member of the Dana-Farber/Harvard Cancer Center, designated a comprehensive cancer center by the National Cancer Institute. It provides adult cancer care with Brigham and Women's Hospital as Dana-Farber/Brigham and Women's Cancer Center and it provides pediatric care with Boston Children's Hospital as Dana-Farber/Boston Children's Cancer and Blood Disorders Center. Dana-Farber is the top ranked cancer center in New England, according to U.S. News & World Report, and one of the largest recipients among independent hospitals of National Cancer Institute and National Institutes of Health grant funding.
Source-Newswise