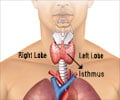
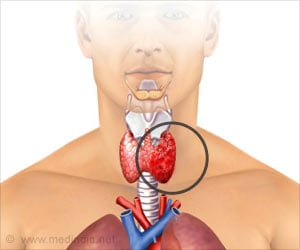
Oral anti-angiogenic therapy lenvatinib has shown dramatic improvement in progression-free survival in patients with advanced radioiodine-refractory thyroid cancer.
The international, randomized, Phase III double-blind study enrolled 392 patients who had progressive, refractory disease. Patients were randomized at a 2:1 ratio to receive either the study drug or placebo, respectively. 261 received lenvatinib and 131 received a placebo. At the time of disease-progression, patients in the placebo group could receive lenvatinib. The primary endpoint of the study was progression-free survival; secondary endpoints tested response rate, overall survival and safety.
Participants who received the study drug, the median progression-free survival rate was 18.3 months, compared with 3.6 months in those who received placebo. The overall response rate in the study arm group was 64.8 percent (with 4 complete and 165 partial responses), and 1.5 percent in the placebo arm. The median overall survival was not reached in either of the group. For patients with advanced and metastatic disease, lenvatinib and this class of drugs is the first potential for improved overall survival.
Sherman added, "In our study, we not only saw a dramatic improvement in progression-free survival, there was also a 65 percent response rate - almost unprecedented results for thyroid cancer patients with such advanced disease. We also found a strongly suggestive trend in how long patients lived, and a small number of patients had a complete response. While we couldn't identify tumor mutations that might predict response, this represents a very exciting area of study going forward in hopes of possibly offering cure to a greater number of patients. The drug is not without side effects - more than 40 percent of patients that received lenvatinib experienced some reaction, with hypertension being the most common, but could be managed. Other side effects included: diarrhea, fatigue, nausea, decreased appetite and weight; 37 patients discontinued the drug because of adverse effects. Also, six of 20 deaths that occurred during the treatment period were determined to be drug-related by their treating physicians. The side effect profile is actually quite typical for this class of drugs. We've learned over the years to be aggressive about dosing modifications and coming up with clever ways of helping patients tolerate the medication where drug effectiveness is maintained but with a minimum of those side effects. It's paramount that patients are selected carefully and physicians giving the drug focus on symptom support."
Several other studies with lenvatinib are in development, including in other types of thyroid cancers, and in combination with other novel therapies for radioiodine refractory patients.
The study is published in the 'New England Journal of Medicine'.
Advertisement