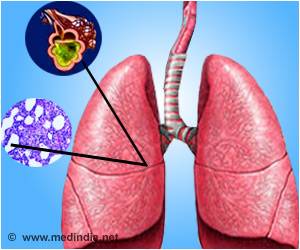
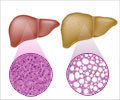
Acute liver failure cases dropped after the Food and Drug Administration rule to lower the dosage of acetaminophen
Acetaminophen Dosage
The challenge was that too-high doses of‘The FDA mandate that confines acetaminophen dosage to 325 milligrams per tablet in combination acetaminophen-opioid medications was related with a decline in the yearly rate of hospitalizations acute liver failure cases’





To examine the effect of this change, researchers in the JAMA study looked at yearly rates of hospitalization and acute liver failure cases in two independent, contemporaneous data sources, the National Inpatient Sample, or NIS, and the Acute Liver Failure Study Group, or ALFSG. The NIS is a very large U.S. hospitalization database with more than 473 million hospitalizations from 2007 to 2019. The ALFSG is a prospective, 32 U.S. medical-center cohort of adult patients with acute liver failure from 1998 to 2019.In each data source, Locke and colleagues found similar declines in the yearly rates of hospitalization and acute liver failure cases associated with acetaminophen-opioid medications after the mandate. They also compared toxicity seen from acetaminophen-opioid medications versus toxicity from acetaminophen alone. In contrast to the declines from acetaminophen-opioid medications after the mandate, rates of hospitalization and acute liver failure cases associated with acetaminophen alone - where the dosage is not constrained by the FDA — continued to rise after the combination drug mandate.
The detailed results looked at four groups: NIS
As an example of detailed findings, in the NIS group, the predicted incidence of hospitalizations associated with acetaminophen-opioid toxicity one day prior to the FDA announcement was 12.2 cases per 100,000 hospitalizations. By Q4 2019, it was 4.4 cases per 100,000 hospitalizations. The odds of a hospitalization involving acetaminophen-opioid toxicity increased 11 percent per year before the announcement and decreased 11 percent per year after the announcement.
In the ALFSG group, the predicted percentage of acute liver failure cases from acetaminophen-opioid toxicity one day prior to the FDA announcement was 27.4 percent. By Q3 2019, it was 5.3 percent. The percentage of acute
Advertisement
Advertisement