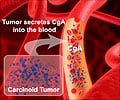
Lutathera, a newly approved drug, provides improved quality of life for midgut neuroendocrine tumor patients while enhancing progression-free survival. The drug consists of a somatostatin analog combined with a radioactive isotope that directly targets neuroendocrine tumor cells.
‘Lutathera, a newly-approved drug may provide improved quality of life for midgut neuroendocrine tumor patients.’





Dr. Jonathan Strosberg, head of Neuroendocrine Tumor Program at Moffitt "Treatment options have been limited for patients with neuroendocrine tumors and toxicities of treatment can often outweigh the benefit. Our studies have shown Lutathera is an effective option to treat tumor progression and also provide patients with a better quality of life," said Jonathan R. Strosberg, M.D., head of the Neuroendocrine Tumor Program at Moffitt Cancer Center. The Journal of Clinical Oncology published new data from the NETTER-1 clinical trial highlighting the impact of Lutathera on patients' quality of life. Strosberg notes the importance of this issue, given the relatively long durations of treatments and overall survival compared to other malignancies. The results showed that treatment with Lutathera provides significantly longer time to deterioration of quality of life for patients compared to those treated with octreotide LAR alone.
Patients in the study were given questionnaires every three months until tumor progression. Quality of life was measured by global health status, physical functioning, role functioning, fatigue, pain, diarrhea, disease-related worries and body image. The time of deterioration was defined as the period of time from the patient's first questionnaire response until their quality of life score declined ten or more points.
Patients treated with Lutathera reported improvement in many of the typical side effects, such as fatigue, pain and diarrhea. Differences in the median time to deterioration were 28.8 months versus 6.1 months for global health status, and 25.2 months versus 11.5 months for physical functioning.
Source-Eurekalert