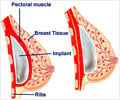
Outcome reporting in breast reconstruction needs to improve in its standards, according to a review published online December 3rd in The Journal of the National Cancer Institute.
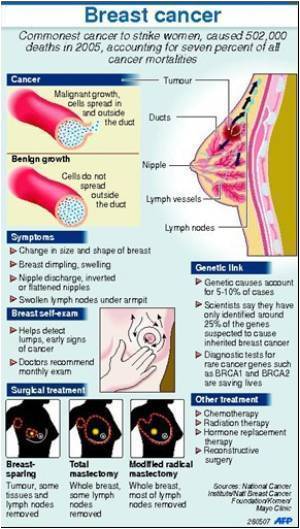
To summarize the reporting standards of surgical outcomes in breast reconstruction, Shelley Potter, M.D., of the University of Bristol, and colleagues, reviewed 134 studies reporting surgical outcomes of breast reconstruction involving over 42,000 women. Over half of the studies, or 55%, were cohort studies, 36.6% were case series studies, and 8.2% were randomized controlled trials. Specifically, the researchers looked at prospective or retrospective accrual of data, duration of follow-up, proportion of complications, reporting of total and procedure-specific complications, severity of complications, length of hospital stay and adjustment for risk factors such as smoking or radiotherapy.
The researchers found an overall dearth of reporting and inconsistency in the data regarding outcomes of breast reconstruction. The studies defined clearly fewer than 20% of the complications they reported, and only half the studies considered risk factors for adverse outcomes. The authors write, "Details such as the severity of complications (41.8% of all studies), duration of follow-up (58.2%) and overall complication rates (59.7%) were often omitted." Furthermore, many studies had important methodological problems.
The authors write that their review has indicated the need for a standardized approach to outcome assessment in breast reconstruction that also includes non-clinical factors. They write, "Traditional clinical outcomes remain important, but patient-reported outcomes such as satisfaction, body image, functional results, and cosmetic outcome will also need to be incorporated if the outcomes selected are to be of value to the women making decisions about reconstruction."
In an accompanying editorial, Monica Morrow, M.D., and Andrea L. Pusic, M.D., of Memorial Sloan-Kettering Cancer Center, write that the review points to the need for improved outcome reporting as scientists continue to search for therapies. The editorialists write, "In contrast to the search for new breast cancer therapies to improve survival, a long-term and enormously expensive task, improved standards for outcome reporting for reconstruction, and other aspects of breast cancer treatment have the potential to improve patient quality of life in the short term at a relatively modest cost."
Advertisement