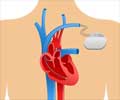
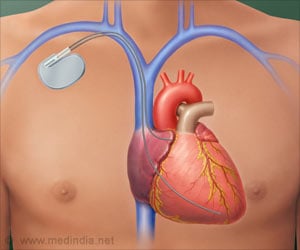
The Advisa single chamber pacemaker is the latest addition to a growing number of Medtronic devices that are designed for MRI access.
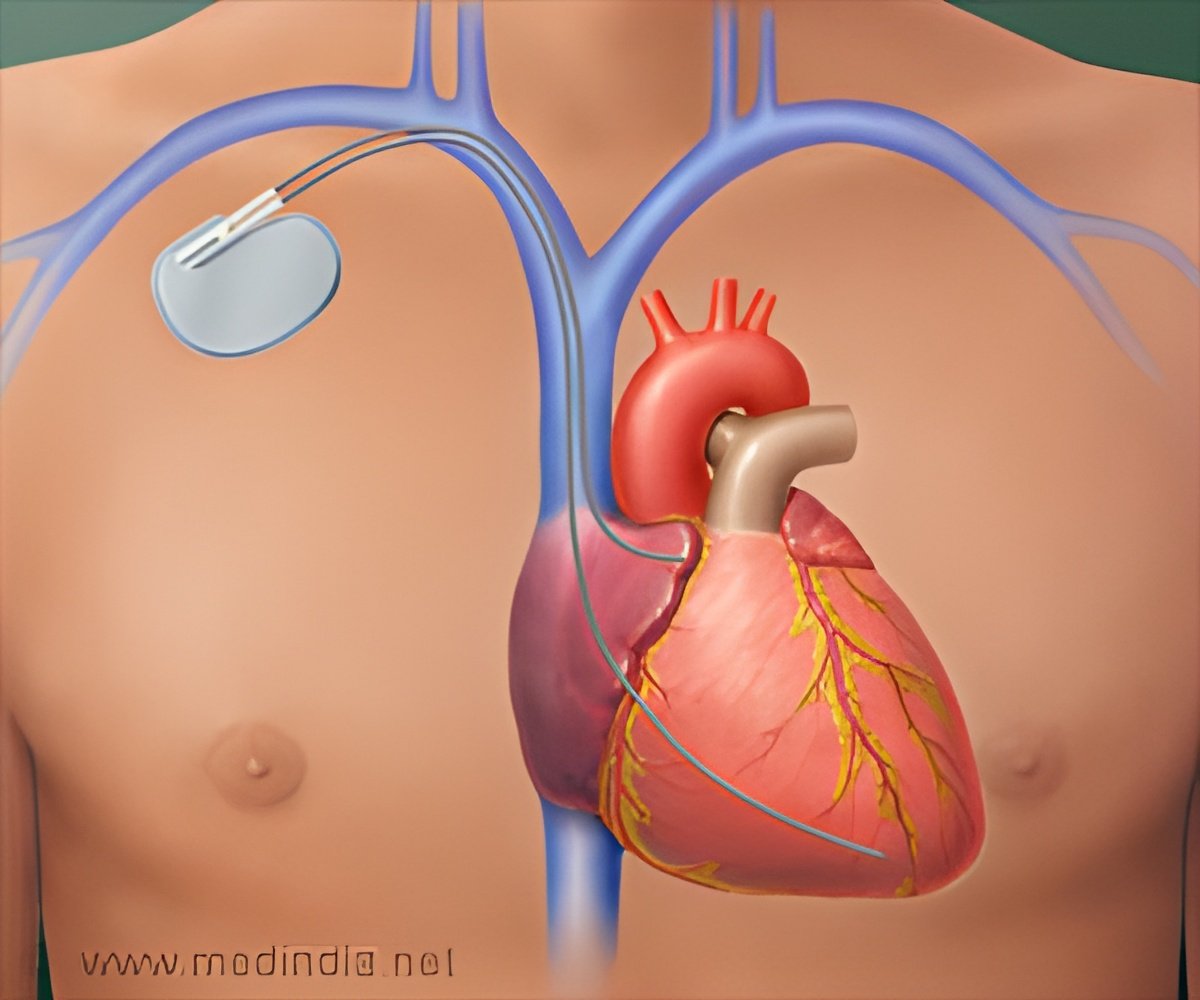
The Advisa SR MRI has been updated with additional information management and storage, with a battery life 35 longer than the Adapta pacemaker and compatibility with MR scans.
“MRI is a vital diagnostic tool that was not available to pacemaker patients before Medtronic released the world’s first MR-conditional pacing system in 2008,” said Brian Urke, Vice president and general manager of the bradycardia business at Medtronic.
Medtronic MR-conditional pacing systems have been evaluated in four clinical studies with more than 3,600 patients and offer the most extensive portfolio of MR-conditional pacing devices.
The Advisa single chamber pacemaker is the latest addition to a growing number of Medtronic devices that are designed for MRI access.
Source-Medindia