The US Food and Drug Administration on Monday approved the first pacemaker system -- produced by medical device giant Medtronic Inc.

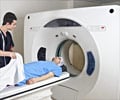
Medtronic estimates that about 200,000 US pacemaker patients opt out of MRI scans every year even though they play a critical role in making a wide range of health diagnoses.
Its Revo MRI SureScan Pacing System has a function that can be turned on before a scan in order to prepare patients for the MRI machines, which can be up to 30,000 times more powerful than the Earth’s magnetic field.
The function reduces or eliminates potential MRI hazards.
J. Rod Gimbel of Cardiology Associates of East Tennessee in Knoxville said the new pacemaker was "a major technological breakthrough for patients who need access to MRI."
"Providing pacemaker patients with access to MRI allows detection and treatment of serious medical conditions such as stroke, cancer and a wide variety of important neurologic and orthopedic conditions."
Advertisement
"FDA’s approval of the Revo pacemaker represents an important step forward toward greater device innovation," said Jeffrey Shuren, director of the FDA’s Center for Devices and Radiological Health.
Advertisement
The FDA is requiring cardiologists and radiologists who use the system to receive appropriate training.
Source-AFP