Lung cancer patients who were given 2mg of Keytruda had longer overall survival compared with those taking docetaxel, a standard treatment for lung cancer.
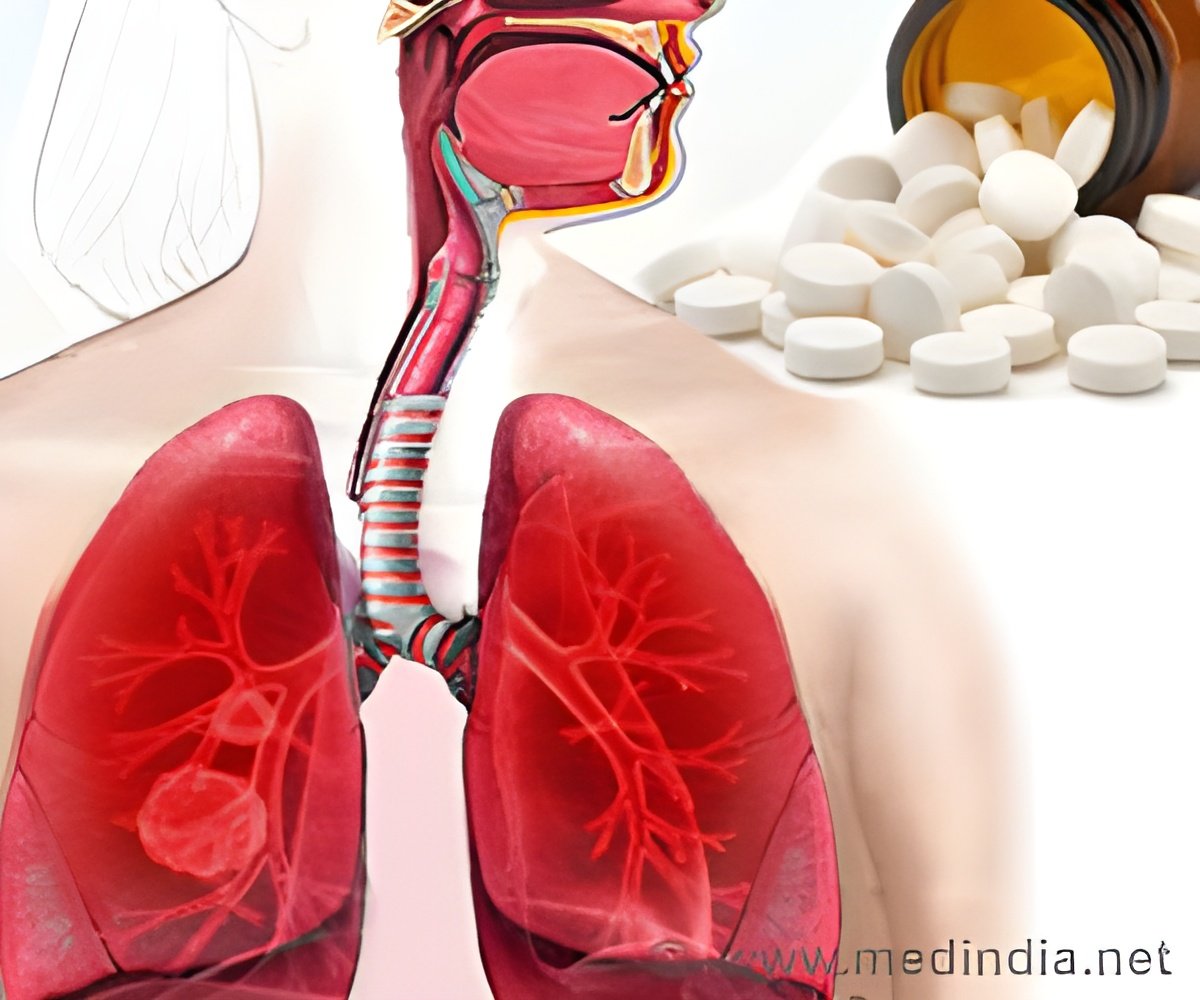
‘The drug Keytruda is used for treatment of Lung Cancer and acts by enabling the patient''s own immune system to recognize and therefore attack the cancer.’





Researchers say that patients whose tumors have high levels of PD-L1, a protein linked to increased risk of the disease, had longer survival to chemotherapy without progression of the disease. This drug will be used for patients with metastatic or advanced lung cancer who did not respond to previous treatments.Safety of Keytruda was consistent with what had been seen in previous trials among lung cancer patients, Merck said in a release that included only summary "topline" information from the results.
More detailed data from the study will be provided soon, Merck said, adding that it will ask the U.S. Food and Drug Administration later this year to add the new data to the drug's package insert label.
Upon approval of this drug earlier this month, the trial showed that it shrank the tumor cells of patients who possess faulty PD-L1 by 41 percent with effects lasting for up to nine months. "Our growing understanding of underlying molecular pathways and how our immune system interacts with cancer is leading to important advances in medicine," FDA spokesperson Dr. Richard Pazdur said when they approved the drug.
"Today's approval of Keytruda gives physicians the ability to target specific patients who may be most likely to benefit from this drug," he added.
Advertisement
Source-Medindia