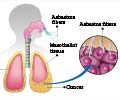
Combining two cancer immunotherapy drugs helps prolong survival in aggressive cancer malignant mesothelioma patients. The drugs include a novel immune modulator and one that focuses and activates the antitumor immune response.
‘Use of two cancer immunotherapy drugs can reduce tumor size and prolong survival in mesothelioma cancer patients.’





In their report published in Cancer Immunology Research, a team from the Vaccine and Immunotherapy Center (VIC) at Massachusetts General Hospital (MGH) describes how adding AMD3100 (plerixafor) - previously approved for the stimulation of stem cell production prior to bone marrow transplantation - to their investigational drug VIC-008 more than doubled the animals’ survival time. Among the mechanisms identified as underlying the combination treatment’s effects was changing a population of immunosuppressive T cells into a type that could enhance an antitumor immune response.
"Mesothelioma, a tumor that is caused by asbestos exposure, has been extremely hard to treat; and patients usually survive only 12 to 18 month after diagnosis," says Mark Poznansky, MD, PhD, director of the MGH-VIC and senior author of the report.
"Since the advent of cancer immunotherapy, people have tried to apply immunotherapeutic drugs to mesothelioma with limited success. We are very excited at the prospect that this drug combination may be much more effective in prolonging patients’ lives."
The study is the result of a collaboration between Poznansky and Jeffrey Gelfand, MD, an MGH-VIC investigator who had developed VIC-008 - also called Jantibody, in memory of Gelfand’s wife Janet who succumbed to ovarian cancer - as a potential treatment for ovarian cancer.
Advertisement
While previous research had shown some effectiveness of this molecule in a mouse model of ovarian cancer, that benefit was limited by the immunosuppressive environment within tumors, particularly the presence of regulatory T cells (Tregs). The current combination appears to overcome this limitation.
Advertisement
While research has supported AMD3100’s ability to inhibit tumor growth and metastasis, the mechanism behind its effects on mesothelioma had not been determined. Since expression of CXCR4 and CXCR12 are increased as mesothelioma progresses, VIC researchers investigated the potential of combining AMD3100 with VIC-008 to treat these tumors.
Their series of experiment in two different mesothelioma mouse models found the following:
- While single-agent treatment with either drug had limited effects against mesothelioma, treatment with both drugs significantly reduced tumor size and prolonged the animals’ survival.
- Treatment with VIC-008 increased lymphocyte infiltration of tumors and both the levels and the anti-tumor response of CD8 T cells, which kill damaged or infected cells.
- AMD3100, alone or in combination with VIC-008, decreases expression of the immune checkpoint molecule PD-1 on CD8 T cells, implying that the CXCR4/CXCR12 pathway modulates PD-1 expression.
- AMD3100, alone or in combination, reduced the number of tumor-infiltrating Treg cells and increased the proportion of CD8 T cells.
- AMD3100 further reduces immune suppression by shifting characteristics of Tregs toward those of helper T cells, which would enhance antitumor effects.
Chen adds, "We believe that we can find an approach that combines Jantibody and AMD3100 to regroup and redirect immune responses in order to combat cancer." He is an instructor in Medicine at HMS.
Gelfand, a senior scientist at VIC and professor (part-time) at HMS, says, "AMD3100 is already an FDA-approved drug, whose use and application the MGH VIC is hoping to extend in various cancers. Jantibody focuses the AMD3100-invigorated immune response on critical tumor structures, markedly enhancing tumor control. We hope these data will help to move Jantibody closer to human tumor therapy, fulfilling one of my wife’s last wishes."
Source-Eurekalert