metastatic melanoma can be treated by genetically modified virus injection that induces the body's own immune cells to kill the cancer cells.
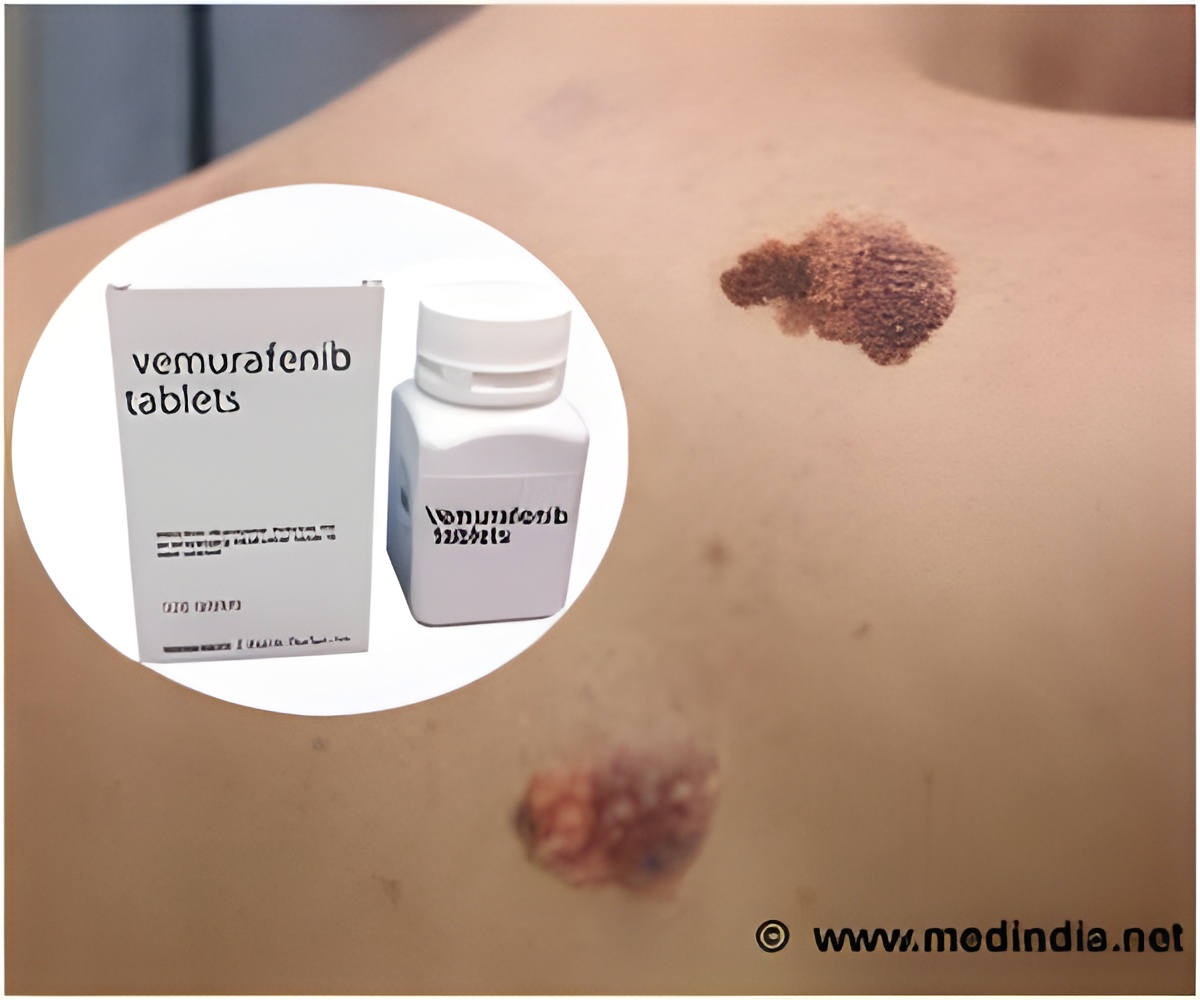
‘The classical chemotherapy treatment method is cytotoxic that not only kills cancer cells, but it also destroys healthy cells. However, the TVEC therapy induces the immune system to kill only tumor cells.’





The researchers evaluated 80 adult patients treated with talimogene laherparepvec (TVEC, sold under the brand name Imlygic, by AMGEN Inc.) over a three-year period ending October 1, 2018. The Food and Drug Administration approved TVEC in 2015 for the treatment of stage IIIB to advanced-stage IV metastatic melanoma. TVEC is a genetically modified herpes virus that contains the stimulatory factor known as GM-CSF that increases a tumor-specific immune response. TVEC is injected directly into the skin tumor in the physician's office or clinic and the patient may leave immediately afterward, without the severe side effects of chemotherapy or other cancer drugs that act on the body's immune system. The stimulatory factor sends a signal to attract white blood cells into the tumor, Dr. Ollila explained, thereby inducing the body's activated immune system to kill the metastatic melanoma cells.
The researchers reported that 46 percent of participants (37 patients) had stage IIIB disease, 31 percent (25) had stage IIIC disease, 1 percent (1) had clinical stage IIID disease, and 20 percent (16) had cancer that spread to a distant site at the time of treatment. Patients received a median of five cycles of TVEC. Most study patients--57 percent--received some form of therapy before enrollment in the study.
The researchers found that 39 percent of participants (31 patients) had a complete local response to TVEC therapy, and 18 percent (14 patients) had a partial response. "It's pretty hard to ignore a response rate of 39 percent," Dr. Ollila said. He noted that patients with stage IIIB disease had an even higher complete local response rate of 68 percent, compared with those with higher-stage disease: 26 percent for IIIC, 0 percent for IIID, and 6 percent for IV. At a median follow-up of 12 months, 59 percent of patients with stage IIIB disease showed no signs of recurrent disease.
Dr. Ollila noted that these findings surpass those from the clinical trial that led to the approval of TVEC. The overall response rate from the clinical trial was 26.4 percent.* However, that trial included a higher percentage of patients with cancer that had spread to other organs, including the lungs.
Advertisement
One key takeaway from the study was that patients with stage IIIB/C or stage M1A metastatic melanoma may benefit more from the drug than those with higher stage cancers, Dr. Ollila said. "We reported a complete response rate that was higher than the 2015 clinical trial because the clinical trial included higher-risk patients in whom TVEC may have limited effectiveness," he said.
Advertisement
Besides chemotherapy, another existing treatment for skin cancer is surgical removal of the tumor, but that too can be problematic, Dr. Ollila said. "We know that surgeons will eventually reach a point where surgical resection is no longer feasible." The most compelling finding of the study is that it supports TVEC injection as an option for melanoma. "It appears that there's no role for traditional chemotherapy any longer for treating this disease," Dr. Ollila added.
Source-Eurekalert