MiCheck™ technology using Glypican-1, a biomarker that detects prostate cancer will be introduced at the International Conference of Urology in New Orleans.
Trial investigator, Dr Jonathan Henderson, will present full data from Minomic’s recent pilot study of the MiCheck™ technology on 300 patient samples collected from 10 key urology research centres within the CUSP network, located with geographical diversity throughout the United States.
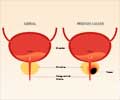
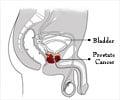
Dr Henderson has worked closely with the principal investigator for the study, Dr Neal Shore. The non-randomised pilot study was designed to further verify the accuracy and reliability of the technology in differentiating normal, benign and prostate cancer samples. The MiCheck™ technology uses Glypican-1 (GPC-1), which is a newly identified biomarker in prostate cancer.
GPC-1 shows clinical utility in improving specificity of detection for prostate cancer when comparing both prostate cancer patients to those patients who are otherwise healthy or who have documented benign prostatic disease.
Minomic Chief Executive Officer Dr Brad Walsh said the American Urology Association presentation was a critical platform to highlight the company’s lead technology, particularly in the high value United States market.
He commented, “This event draws together key opinion leaders in urology from around the world. Presenting our technology at this important showcase is a terrific opportunity. We look forward to presenting full data from the recent successful pilot study, which has shown that MiCheck™ is twice as specific as standard prostate cancer screening technologies.”
Advertisement
Dr Shore said he was pleased to continue as principal investigator “A subsequent pivotal trial of the MiCheck™ technology will be launched in the United States later this year, with clinicians/researchers screening 1200 patient samples to provide final stage validation. These results, combined with results from this 300 patient study, will be evaluated and subsequently included in a comprehensive data package submitted to the Food and Drug Administration for regulatory approval.”
Advertisement

![Prostate Specific Antigen [PSA] & Prostate Cancer Diagnosis Prostate Specific Antigen [PSA] & Prostate Cancer Diagnosis](https://images.medindia.net/patientinfo/120_100/prostate-specific-antigen.jpg)